The U.S. Food and Drug Administration (FDA) authorized the Tidepool Loop, an automated insulin dosing app intended for the management of type 1 diabetes (T1D). The Loop is, essentially, an algorithm that can, eventually, be used to work with commercially available insulin pumps and continuous glucose monitors (CGMs). The goal of the interoperable design: Provide flexibility for users and their healthcare teams to choose the compatible components that work best for them in managing their care.
It will be available as an app on iOS, to enable insulin delivery from a compatible Apple Watch. Tidepool has not yet announced its initial launch device partners, but the company has a development partnership with Dexcom and additional yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform.
Breakthrough T1D Impact
Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with type 1 diabetes have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation. To date, Breakthrough T1D has funded more than $140 million in artificial pancreas research.
Through these grants, Breakthrough T1D supported the development of the algorithm and other open source programs—in partnership with the Helmsley Charitable Trust—through grants to Tidepool.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
Here is Aaron J. Kowalski, Ph.D., announcing the approval, highlighting Breakthrough T1D’s role in the research and regulatory process, and reiterating that people with T1D should connect with their physician about the treatments best for them:
To learn more, visit the Tidepool Loop website.
The Food and Drug Administration (FDA) authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system, for children ages 2+. It includes an algorithm placed in a waterproof, closed loop insulin pump and communicates directly with a Dexcom G6 continuous glucose monitor (CGM).
There were three FDA approvals of artificial pancreas systems on the market: The Medtronic 670G, approved in 2016, the Tandem Control-IQ™, approved in 2019, and the Medtronic 770G, approved in 2020. This marks the first tubeless hybrid closed loop system to receive FDA authorization.
Safe and effective system use was demonstrated in preschool children aged 2+ with T1D during a 3-month pivotal study. This year, we reported on the extension study, to evaluate if glycemic outcomes continued at 12 months, presented by Daniel DeSalvo, M.D., who had a Breakthrough T1D postdoctoral fellowship from 2014-2016 with world-renowned researcher Bruce Buckingham, M.D., at the American Diabetes Association’s 82nd Scientific Sessions. At 12 months, these children had lower A1c and greater time-in-range, and there was no DKA or severe hypoglycemia, indicating the potential long-term benefit of the Omnipod 5 in very young children with T1D.
For more information on it’s use for 6+, visit our blog, FDA Authorizes a Fourth Artificial Pancreas System.
Breakthrough T1D Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with increasingly advanced versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with type 1 diabetes have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The Breakthrough T1D Artificial Pancreas Project and the Breakthrough T1D Artificial Pancreas Consortium have dramatically accelerated progress by bringing academic researchers, government agencies, industry, and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
Breakthrough T1D has funded over $140 million to date in artificial pancreas research.
- Breakthrough T1D supported work in this area through investigators in the Breakthrough T1D Artificial Pancreas Consortium, Francis (Frank) Doyle, Ph.D., Eyal Dassau, Ph.D., and Howard Zisser, M.D., and their colleagues at the Sansum Institute (California). They created the first algorithm, which was eventually licensed by Insulet Corporation that led to the development of Omnipod 5.
- Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system in 2016, the first approved artificial pancreas system.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
For individuals who use the Tandem t:slim X2™, bolusing from their insulin pumps just got a bit easier.
On February 16, 2022, Tandem announced the FDA clearance of bolus insulin dosing from their t:connect® mobile app. Soon, individuals with the Tandem t:slim X2™ pump will be able to bolus remotely from either their iOS or Android smartphone. For people who keep their insulin pump in hard to access places, it’s a big deal.
“This is a gamechanger for me personally,” says Alecia Wesner, who lives with T1D. “I often wear clothing without any easily accessible places to keep my pump. That means I often store my pump on my bra or in my tights—which makes it hard to make the many daily boluses required to keep my blood sugar in range. This feature is simply going to make it easier for me to live with type 1 diabetes.”
Better Devices, Better Outcomes
People with T1D need better tools if they’re going to do better—that means achieving an optimal HbA1c, increased time-in-range, and more. This clearance makes the t:slim X2™ a better tool.
The t:slim X2™ with Control-IQ™ technology is an automated insulin delivery (AID), or artificial pancreas, system. This system is comprised of a Dexcom G6® continuous glucose monitor (CGM), the t:slim X2™ pump, and Control-IQ™ technology, which is the algorithm that automatically administers insulin on behalf of the user. AID systems have demonstrated they improve outcomes for people with T1D—but there are still barriers to adoption. These include ease of wear, comfort, and user interface.
This new feature potentially removes a barrier to adoption, meaning that more people will hopefully take advantage of this life-changing technology.
“Breakthrough T1D supports AP systems not just for their clinical benefits, but also for their ability to improve quality of life,” says Jonathan Rosen, Ph.D., Breakthrough T1D Associate Director, Research. “The FDA clearance of bolus insulin dosing on the t:slim X2 pump via the t:connect mobile app is great for the T1D community, since people will now be able to conveniently and discreetly bolus insulin from both iOS and Android smartphones.”
A Long History of Breakthrough T1D Support
Supporting research into better therapies for T1D has been a Breakthrough T1D research priority for decades. Breakthrough T1D founded the Artificial Pancreas Project in 2005 and the Breakthrough T1D Artificial Pancreas Consortium, which have dramatically accelerated progress by bringing academic researchers, government agencies, industry, and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
In addition to playing a leadership role in the field, Breakthrough T1D funded grants that supported the initial development of the Control-IQ™ technology, worked with the FDA to establish a regulatory protocol for AID systems like the t:slim X2™ with Control-IQ™, and, crucially, tirelessly advocated for the Special Diabetes Program (SDP), which funded the clinical trial that provided data for the 2019 FDA approval of Control-IQ™ .
While this is a notable step forward, Breakthrough T1D will continue working to improve therapy options for the T1D community.
Tandem plans for a limited rollout of this feature in the spring with an expanded release in the summer.
The Food and Drug Administration (FDA) authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system for individuals 6 and older. It includes an algorithm placed in a waterproof, closed loop insulin pump and communicates directly with a Dexcom G6 continuous glucose monitor (CGM).
There were three FDA approvals of artificial pancreas systems on the market: The Medtronic 670G, approved in 2016, the Tandem Control-IQ™, approved in 2019, and the Medtronic 770G, approved in 2020. This marks the first tubeless hybrid closed loop system to receive FDA authorization.
Data for Insulet’s submission of Omnipod 5 came from their Omnipod 5 Automated Insulin Delivery System pivotal trial. The trial included 128 adults and adolescents (14–70 years old) and 112 children (6–<14 years old) and demonstrated improvements in several key metrics, including a remarkably low rate of hypoglycemia:
- Adults and adolescents:
- Time in range (70–180 mg/dL) increased 9% from 65% to 74% compared to participants’ standard therapies (an additional 2.2 hours per day)
- Average HbA1c decreased from 7.16% to 6.78%
- Median time below 70 mg/dL decreased from 2.0% to 1.1%
- Children:
- Time in range (70–180 mg/dl) increased 15% from 53% to 68% compared to participants’ standard therapies (an additional 3.7 hours per day)
- Average HbA1c decreased from 7.67% to 6.99%
- Median time below 70 mg/dL remained at 1.5%
This data demonstrates that this system will help people with T1D achieve improved glucose control—a key goal of Breakthrough T1D’s Improving Lives program.
Breakthrough T1D Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with increasingly advanced versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with type 1 diabetes have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The Breakthrough T1D Artificial Pancreas Project and the Breakthrough T1D Artificial Pancreas Consortium have dramatically accelerated progress by bringing academic researchers, government agencies, industry, and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
Breakthrough T1D has funded over $135 million to date in artificial pancreas research.
- Breakthrough T1D supported work in this area through investigators in the Breakthrough T1D Artificial Pancreas Consortium, Francis (Frank) Doyle, Ph.D., Eyal Dassau, Ph.D., and Howard Zisser, M.D., and their colleagues at the Sansum Institute (California). They created the first algorithm, which was eventually licensed by Insulet Corporation that led to the development of Omnipod 5.
- Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system in 2016, the first approved artificial pancreas system.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
Per Insulet, “Omnipod 5 is expected to be broadly available shortly after the limited market release.”
Hybrid closed loop or artificial pancreas systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an algorithm that automatically administers insulin. And a new paper published in the New England Journal of Medicine demonstrates that hybrid closed loop systems help children as young as 1 year old achieve better glycemic control.
This study, led by Roman Hovorka, Ph.D., and funded in part by Breakthrough T1D, compared data from children ages 1-7 on hybrid closed loop therapy to a CGM and insulin. The data showed conclusively that the children on the hybrid closed loop therapy did better.
Breakthrough T1D has funded over $135 million in artificial pancreas research to date, in addition to working tirelessly to ensure these systems have a reasonable pathway to regulatory approval and are covered by payers.
More Proof Artificial Pancreas Systems Work
There have been many, many studies examining the benefits of artificial pancreas systems on glycemic control in a myriad of patient populations. These studies, several of which have been funded by Breakthrough T1D, have led to the development and commercialization of multiple systems with more on the immediate horizon.
This study is yet another to show the benefits. Children on the hybrid closed loop system spent an average of 8.7 percent more time-in-range each day compared to the control group. That’s over two hours every day! Children on the system also had lower HbA1cs and without increased hypoglycemia.
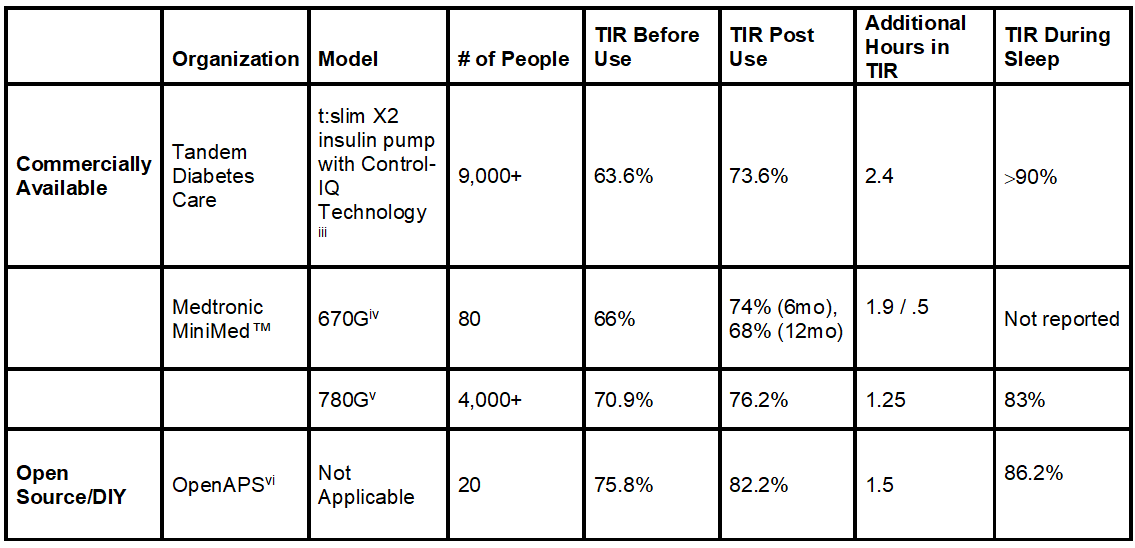
Artificial Pancreas Systems: What’s Available?
Led by Breakthrough T1D, the past 20 years have seen a flurry of innovation and investment in artificial pancreas research. As this technology continues to advance, more patient populations will have access to and benefit from these therapies. In Europe, CamAPS—the artificial pancreas system in this publication—is approved for ages 1 and up and the Medtronic 780G is approved for users between ages 7 and 80 years old. In the United States, the Medtronic 770G is the only automated insulin delivery system approved for children as young as two, and the Tandem Control-IQ™ is approved for individuals 6 and up. (There is a Control-IQ™ clinical trial currently recruiting in the United States, studying these systems in children ages 2-6.)
Type 1 diabetes (T1D) and sleep, or lack thereof, are often tied together. Someone living with T1D must navigate a wide range of challenges that can impact sleep, and poor quality of sleep negatively affects your psychological and physical health.
In addition, one of the greatest fears of nighttime is low blood sugar (called hypoglycemia), which occurs when blood-sugar levels fall below 70 mg/dL. Symptoms can include shaking, an accelerated heart rate, and clammy skin. It’s possible to sleep through a hypoglycemic event, but you can wake up tired, confused, sweaty, and can feel lethargic or foggy.
But artificial pancreas systems are changing that.
Artificial pancreas systems (also called automated insulin delivery systems) have two interrelated functions: Monitoring the blood-sugar levels in your body (through a continuous glucose monitor) and automatically providing insulin to keep them in a healthy range (through an insulin pump), via a smart algorithm. By automating much of blood-sugar management, these systems can help improve diabetes-related outcomes and lighten the burden of T1D.
Even during sleep.
Time-in-Range and Sleep
Time-in-range (TIR) is a measurement that tells you what percentage of the day your blood sugars are in your goal range (typically 70-180 mg/dl). The American Diabetes Association recommends that people with diabetes spend 70% of their time in the target range, meaning you should aim for roughly 17 out of 24 hours each day to be in range. Unfortunately, less than half of the diabetes population meets this recommendation.[i]
But experts emphasize that even a 5% improvement in TIR—let’s say, going from 60% to 65%—is meaningful, as it translates to one more hour per day spent in-range.[ii]
Now, let’s look at some recent scientific findings in the table below.
Not only can the artificial pancreas systems improve time-in-range, but they can dramatically improve time-in-range during sleep.
Note: Left click on image to enlarge.
More time-in-range, even when you’re asleep. Now that’s research that can help you rest easy and more often than not, wake up on the right side of the bed.
Have a goodnight!
Watch Breakthrough T1D’s and Tandem’s Facebook Live event (video below) exploring AP systems and improvements in TIR and sleep.
Editor’s Note: This educational content is made possible with support from Tandem Diabetes Care. Breakthrough T1D produces this content to provide information to our supporters about their potential options for managing their T1D and not as an endorsement of products. Editorial control rests solely with Breakthrough T1D.
RX ONLY. The t:slim X2 insulin pump with Control-IQ technology is indicated for patients with type 1 diabetes, 6 years and older. BOXED WARNING: Control-IQ technology should not be used by people under age 6, or who use less than 10 units of insulin/day, or who weigh less than 55 lbs. For full safety information, visit tandemdiabetes.com/safetyinfo.
[i] Bergenstal RM, Hachmann-Nielsen E, Tarp J, Kvist K, Buse JB. 65-LB: Real-World Continuous Glucose Monitoring Data on Time-in-Range from a U.S. Population, 2015–2019. Diabetes Jun 2021, 70 (Supplement 1); doi: 10.2337/db21-65-LB
[iii] Breton MD, Kovatchev BP. One Year Real-World Use of the Control-IQ Advanced Hybrid Closed-Loop Technology. Diabetes Technol Ther. 2021; 23 (9): 601-608. doi:10.1089/dia.2021.0097
[iv] DuBose S, Bauza C, Verdejo A, Beck RW, Bergenstal RM, Sherr J. Real-world, Patient-Reported and Clinic Data from Individuals with Type 1 Diabetes using the MiniMed 670G Hybrid Closed Loop System. Diabetes Technol Ther. 2021; 10.1089/dia.2021.0176. doi:10.1089/dia.2021.0176
[vi] Lewis DM, Swain RS, Donner TW. Improvements in A1C and time-in-range in DIY closed-loop (Open-APS) users. Diabetes. 2018; 67: 352-OR. https://diabetes.diabetesjournals.org/content/67/Supplement_1/352-OR.abstract.
One of the largest pain points (literally) for people with type 1 diabetes (T1D) is difficulties with infusion sites. They can be uncomfortable, become infected, and need to be changed at least every three days—until now.
The U.S. Food and Drug Administration (FDA) has approved Medtronic’s Extended Wear Infusion Set (EWIS), which is the first infusion set approved for seven days. The EWIS is approved for a significantly longer duration; no other infusion set is currently approved for more than 3 days.
“Infusion sets that last longer, perform better, and are more comfortable to wear are critically important to creating better devices to manage type 1 diabetes,” said Breakthrough T1D Vice President of Research, Sanjoy Dutta, Ph.D. “Breakthrough T1D applauds the FDA for approving Medtronic’s EWIS, which has demonstrated significant benefits that will help people with type 1 diabetes achieve better glycemic control and improve their quality of life.”
EWIS data was presented at the American Diabetes Association 81st Scientific Sessions (listen to Breakthrough T1D CEO Aaron Kowalski, Ph.D., discuss it in his Happy Hour), showing the EWIS is safe and performs better than 2-3 day sets. Aside from the 7-day wear, other positive data include:
- Improved glycemic outcomes for people wearing the EWIS; and
- Increased survival compared to 2-3 days sensors (74.7% to 67.7%).
Breakthrough T1D’s improving lives portfolio is committed to making it easier for people with type 1 diabetes to achieve ideal blood-glucose levels—which includes making wearable devices smaller, better, and more comfortable. Breakthrough T1D has funded several million dollars in research grants to address this specific issue. This includes our initiative on “Extended Wear Infusion Sets” that has funded several projects to unlock the potential of longer lasting disposables. One example is Breakthrough T1D’s partnership with Becton Dickinson to create an extended wear infusion set to be worn for more than 3 days, that was also supported by the Helmsley Charitable Trust. The Breakthrough T1D T1D Fund is an investor in Capillary Bio, a biomedical company developing a novel 7-day infusion set. Learn more about Breakthrough T1D’s efforts in improving lives here.
Learn more from Medtronic.
Last month, SFC Fluidics received a breakthrough device designation for the company’s interoperable insulin delivery pod from the Food and Drug Administration (FDA). The product is in late-stage development and submission for clearance is expected in 2021. The development of SFC’s interoperable pump was partly funded by Breakthrough T1D.
How is the SFC interoperable pump different than other insulin pumps?
SFC Fluidics’ Chief Executive Officer, Anthony Cruz, tells us that the primary goal for SFC’s product development is to empower people with diabetes; to use their cutting-edge microfluidics technology to make things easier and better for the individual.
Two Simple Questions
The Company’s engineers and scientists were challenged with two simple questions, “How do we make the person forget about his/her diabetes?” and “How do we ease the burden of diabetes?”
Insulin pumps currently in use can fall short in dosing accuracy. SFC believes that insulin dose inaccuracy leads to inconsistent diabetes management. Furthermore, current pumps can also fall short in alerting the person of no or low insulin-flow errors—unnecessarily putting millions of people with insulin-dependent diabetes at risk.
SFC’s pump is designed to be safe and deliver highly precise insulin doses. Its pod technology can detect flow or no flow conditions of insulin in real-time, even in extremely small doses.
SFC believes its technology will effectively eliminate 95 percent of over and under dispenses of insulin. The end result promises to be a significant “step up” in peace of mind that the person is safe and can focus on living their lives.
The Breakthrough Device Program is an FDA platform for more effective treatments of serious diseases, like T1D, and enables timely access to these devices by speeding their development, assessment, and review. Other companies that have received the FDA Breakthrough Device designation for T1D technologies include Breakthrough T1D partners EOFlow, Medtronic, and Bigfoot.
Its safety and exceptional dose accuracy are promising features of SFC’s interoperable insulin delivery pod, and Breakthrough T1D applauds SFC on this special honor.
Learn more about how you can support Breakthrough T1D and our work to prevent, treat, and—one day—find cures for T1D.
This week, the Food and Drug Administration (FDA) approved the Medtronic MiniMed 770G artificial pancreas system for use by children aged 2 to 6 with type 1 diabetes (T1D). The 770G System is now approved for ages 2 and older and is the first marketed device that can automatically adjust insulin delivery based on continuous glucose monitoring (CGM) values for children aged 2-6 years.
The Medtronic MiniMed 770G is a Bluetooth-enabled version of the previously approved MiniMed 670G System, which will enable sharing of data on smart devices. While the device automatically adjusts insulin levels, users need to manually request insulin doses to counter carbohydrate consumption at mealtime. (This device is not approved for use in children younger than 2 years old and in individuals who require less than eight units of insulin per day.)
The FDA evaluated data from a clinical trial that included 46 children aged 2 to 6 years old with T1D. The study found no serious adverse events, and data from the study was used to help support the expanded indication for people 2 to 6 years old.
Breakthrough T1D Impact
Breakthrough T1D has been a leader in the development of artificial pancreas systems for 15 years, since starting the Breakthrough T1D Artificial Pancreas Project in 2005 and the Breakthrough T1D Artificial Pancreas Consortium, which have dramatically accelerated progress by bringing academic researchers, government agencies, industry and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with increasingly advanced versions of the device. Manufacturers embraced the roadmap to guide their own research and development programs. Breakthrough T1D has also worked with Congressional leadership—particularly Senate Diabetes Caucus Co-Chairs Susan Collins of Maine and Jeanne Shaheen of New Hampshire and Congressional Diabetes Caucus Co-Chairs Diana DeGette of Colorado and Tom Reed of New York—to secure Federal funding through the Special Diabetes Program (SDP) for artificial pancreas research and overcome obstacles that could delay delivery of artificial pancreas systems to people with T1D.
- Breakthrough T1D has funded over $135 million to date in artificial pancreas research.
- As testing new artificial pancreas technology in people with T1D can be challenging, Breakthrough T1D partnered with the FDA on the regulatory pathway for testing and approval of this technology, leading to the 2012 FDA guidance for artificial pancreas systems. Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system in 2016, the first approved system.
The Special Diabetes Program (SDP) is up for renewal, so if you haven’t done so already, please sign-up to be an advocate, and encourage friends and family to do the same.
- Breakthrough T1D has also been a leading advocate for coverage, affordability and choice for diabetes technology and the insulin people need through our Coverage2Control Campaign. In a victory for the Campaign—driven by our powerful network of advocates—the nation’s largest insurer, United Healthcare, announced that they will expand their insulin pump coverage. People with T1D now have more choice in how they manage their diabetes, and we applaud United Healthcare for this change.
This is the latest example of how Breakthrough T1D research and advocacy work together to make T1D management better and safer. This is a win for the T1D community, and provides people at a young age with T1D another option to improve blood sugar daily management, until cures are found.
A clinical trial at four pediatric diabetes centers in the United States has found that a new artificial pancreas system—which automatically monitors and regulates blood-sugar levels—is safe and effective in children as young as age six with type 1 diabetes (T1D): Tandem’s t:slim X2™ insulin pump with Control-IQ™ technology. The algorithm in the Control-IQ technology came out of work by Breakthrough T1D-funded investigators, Boris Kovachev, Ph.D., and Marc Breton, Ph.D., at the University of Virginia.
This device was initially only authorized by the FDA for use in people 14 and older; the FDA amended its authorization to children 6 and up in June. Results from the trial were published in the New England Journal of Medicine.
“The publication of this landmark study provides proof that artificial pancreas systems work in children at a very young age,” said Sanjoy Dutta, Ph.D., Breakthrough T1D Vice President, Research. “Families now have an additional option to explore as they seek to find the system that best suits their needs, and this is a big win for the T1D community.”
The International Diabetes Closed Loop Protocol-5 (DCLP5) clinical trial recruited 101 children, ages 6-13, to take part, and the results are definitive: Control-IQ helps children with T1D do better. Per the publication, children experienced:
- Increased time in desirable glucose range (70-180 mg/dl) overall (67% for those on Control-IQ compared to 55% in the control group)
- Increased time in range overnight (80% for those on Control-IQ compared to 54% in the control group)
This study was funded by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) with funds from the Special Diabetes Program (SDP), for which Breakthrough T1D is the primary advocate.
Breakthrough T1D has been a leader in the development of artificial pancreas systems since starting the Artificial Pancreas Project 15 years ago. Through years of research funding, collaboration with regulatory agencies and leadership in the field, Breakthrough T1D has helped accelerate the development of transformative therapies that make life better for people living with T1D.
Breakthrough T1D’s goal from the beginning was to support multiple artificial pancreas systems. Now two are on the market, and there are many more in development.
Breakthrough T1D has also been a leading advocate for coverage, affordability and choice for diabetes technology and the insulin people need through our Coverage2Control (C2C) Campaign. In a victory for the C2C Campaign—driven by our powerful network of advocates—the nation’s largest insurer, United Healthcare, announced that they will expand their insulin pump coverage to include the Tandem pump. People with T1D now have more choice in how they manage their diabetes, and we applaud United Healthcare for this change.
Read more about how we are advocating for coverage, affordability and choice.