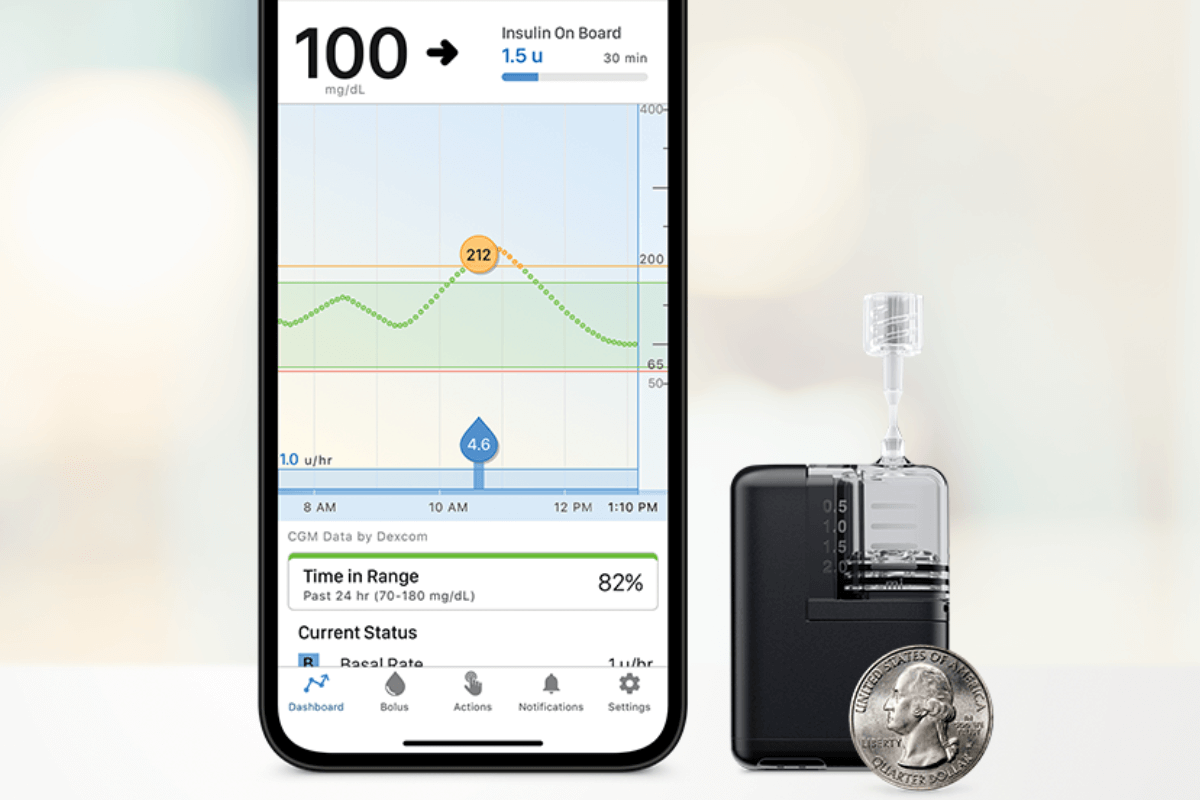
In just the past year, we have had multiple artificial pancreas systems authorized by the Food and Drug Administration (FDA)…and it’s not stopping! Last week, Tandem Mobi—a miniature-sized insulin pump, for use with Tandem’s Control-IQ™ technology and a compatible continuous glucose monitor (CGM)—received FDA clearance.
The Tandem Mobi is half the size of the company’s t:slim X2™ insulin pump, making it the world’s smallest artificial pancreas system. It is fully controllable from a mobile app through a user’s compatible iPhone and is approved for people with diabetes aged 6 years or older
In 2019, Tandem was the second to gain FDA clearance for its Control-IQ™ advanced hybrid closed loop technology, an algorithm that can be used with Tandem’s t:slim X2™ insulin pump and a compatible CGM. It was the first algorithm authorized as an interoperable automated glycemic controller, which means the algorithm could be a component of any open protocol, or interoperable, artificial pancreas system.
From Tandem’s website: A limited release of the Tandem Mobi system is expected to start in late 2023, with full commercial availability expected in early 2024. Current t:slim X2 insulin pump customers, with at least 12 months remaining on their pump warranty, can find more information on how to switch to the new Tandem Mobi through the Tandem Choice Program.
Breakthrough T1D Vision and Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development, and manufacturers embraced this to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project and Consortium almost 20 years ago. Our goal: (1) To ensure that people with T1D would have better, more innovative ways to manage their disease, and (2) to enable a competitive ecosystem that drove continuous innovation.
- Through the project, Breakthrough T1D has dramatically accelerated progress by bringing together academic researchers, government agencies, industry partners, and the Helmsley Charitable Trust to pursue artificial pancreas technology.
Breakthrough T1D has funded over $140 million to date in artificial pancreas research.
- Five algorithm teams of the Breakthrough T1D Artificial Pancreas Consortium and Breakthrough T1D-funded grants made possible the algorithms in the Medtronic MiniMed 670G, 770G, and 780G, Tandem Control-IQ™, CamAPS® FX, Insulet Omnipod 5, Tidepool Loop, and the iLet® Insulin-Only Bionic Pancreas System.
- The Special Diabetes Program, which funds T1D research through the National Institutes of Health, funded the clinical trial that led to Control-IQ’s FDA clearance.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management until cures are found.