Nearly 80 percent of people with type 1 diabetes (T1D) fail to meet HbA1c goals defined by the American Diabetes Association. Despite more widely adopted diabetes technology and an increase in use, there is no improvement in clinical outcomes. This tells us that the current use of insulin alone is not enough, but vTv Therapeutics may have an answer.
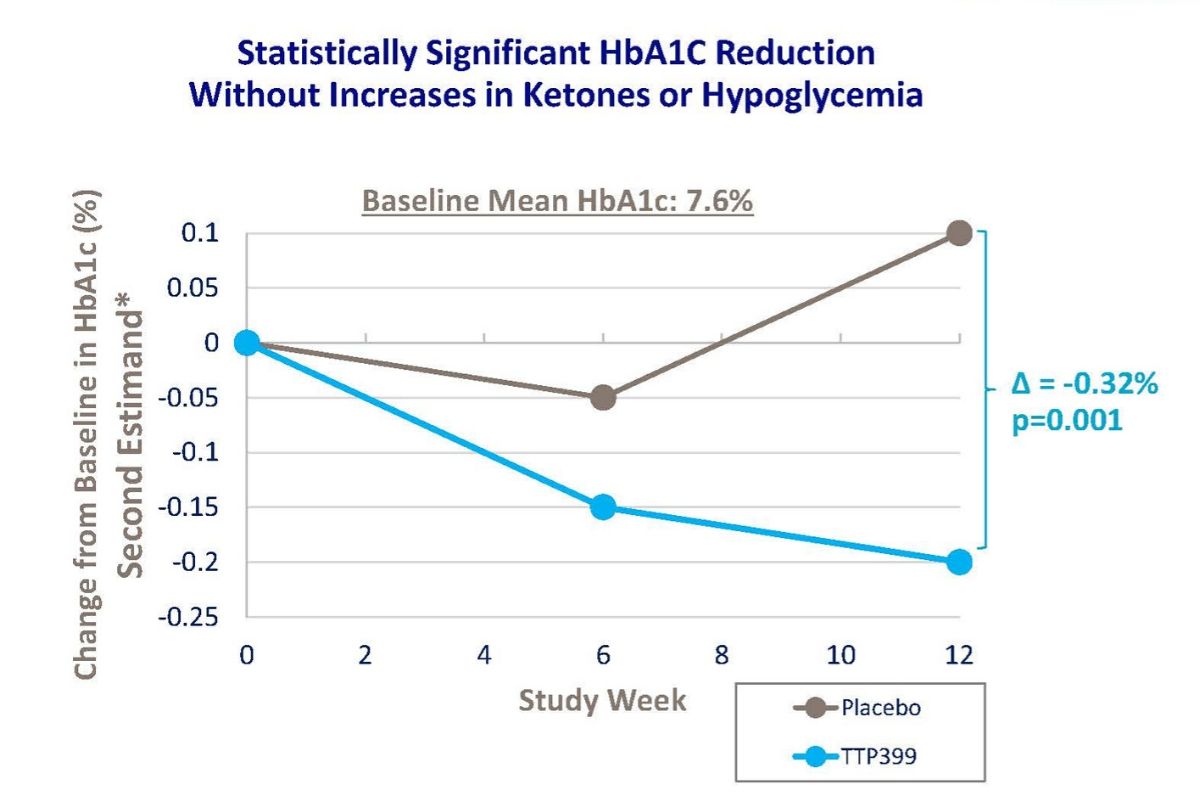
In a Breakthrough T1D-funded clinical trial, they tested the safety and efficacy of TTP399, an oral therapy to be used in conjunction with insulin, in 85 people with T1D. The study successfully achieved its primary objective by demonstrating statistically-significant improvements in HbA1c, compared to placebo, at week 12.
The daily time-in-range was improved by approximately two hours in people treated with TTP399 relative to placebo. TTP399 was well tolerated, and importantly, there were no reports of diabetic ketoacidosis—a complication of T1D—reported in either group. There was no incidence of severe low blood sugar in the treated group and only one incident in the placebo group. People taking TTP399 experienced fewer symptomatic low blood sugar episodes: two subjects taking TTP399 reported at least one event compared to eight subjects in the placebo group (no TTP399).
TTP399 is a glucokinase (or GK) activator. GK acts as a key regulator of sugar levels in the body. If blood glucose levels are deemed too high, activation of GK in the liver has been shown to increase glucose utilization, which in turn lowers glucose levels in the blood.
vTv Therapeutics joined forces with Breakthrough T1D in 2017, to test TTP399 in people with T1D. The positive topline results from this phase II clinical trial follow the positive results obtained in the previous smaller clinical study reported by Breakthrough T1D in June 2019.
The next step: A phase III towards the future path of a registration trial. Stay tuned.