
Updates from the Teplizumab Trial: You’re Not Going to Believe the Results
Last year, at the American Association for Diabetes (ADA) Scientific Sessions, a drug, called teplizumab, was able to significantly delay—for…

Public and Private Organizations, All with the Same Goal: End T1D
In this time of great uncertainty, there is some exciting news out of Europe. Innodia, a partnership between public and…

Dr. Carla Greenbaum Talks about T1D TrialNet During the COVID-19 Pandemic
Carla Greenbaum, M.D., chair of TrialNet—an international Breakthrough T1D-supported network that is dedicated to finding cures for type 1 diabetes…
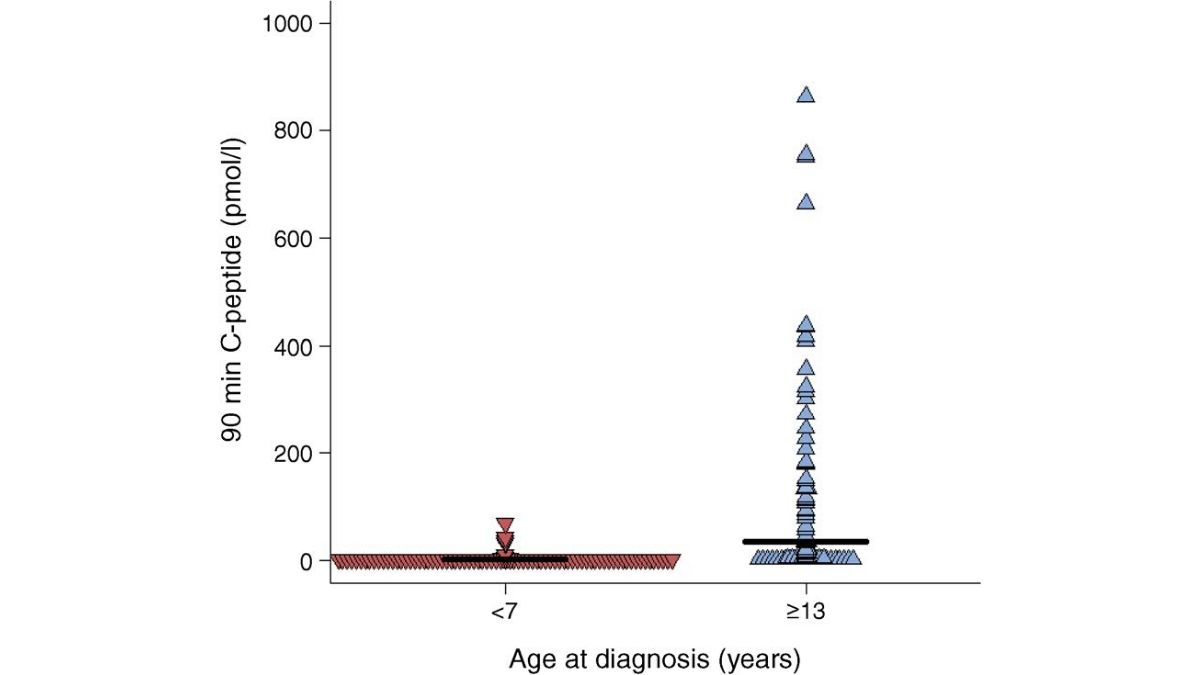
New Research Suggests that T1D Has Two Distinct Types
Breakthrough T1D-funded researchers found that type 1 diabetes is comprised of two different subtypes, called endotypes, which are dependent upon…

Why Does One Scientist Seek Cures for T1D? Answer: Family
Reza Abdi, M.D., has a reason to care about type 1 diabetes research—he has a father and a brother with…

Breakthrough T1D Launches First Center of Excellence, Aiming to Accelerate Type 1 Diabetes Research
Breakthrough T1D today launched its first Center of Excellence, a new research and funding model aimed at accelerating leading type…

FDA Breakthrough Therapy Designation for Teplizumab—Based on the First Study to Delay the Onset of T1D for 2+ Years
The U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to teplizumab, an anti-CD3 monoclonal antibody, for the prevention…

ADA Scientific Sessions Recap—Day #4: Simi Ahmed, Ph.D.
The American Diabetes Association’s Scientific Sessions is almost coming to a close! Here is Simi Ahmed, Ph.D., head of the…

First Human Study to Significantly Delay—by Two Years!—Type 1 Diabetes Onset
This is big. No, wait, this is ENORMOUSLY HUGE. Published today in the New England Journal of Medicine, an immune…

Your Cancer is Gone; Type 1 Diabetes is Here
Immune therapy has transformed cancer care. In some cases, cancer is effectively cured in people with stage 4 disease. But…