
100, 100: Breakthrough T1D Research Leads to a New Class of Glucose-Lowering Agents
You’ve seen the ads: Victoza®. Ozempic®. Trulicity®. But did you know that these drugs came about, in part, because of…
100, 100: Genetically Engineered Human Insulin Created
In 1976, researchers set out to synthesize the human insulin gene and make human insulin, and Breakthrough T1D researchers had…

Insulin Affordability? Yes, Please: Breakthrough T1D Joins Civica to Make Low-Cost Insulin Available to All Americans
In conjunction with leading partners, Breakthrough T1D lends its support to Civica, to help combat a nationwide insulin affordability crisis.
FDA Approval of Senseonics Eversense® CGM for Use for Up to 6 Months
FDA approves Senseonics Eversense® E3, the first long-term implantable CGM system, and the E3 includes technology that extends the use…

Smart Insulin Pen/Smart Cap Bridge Technology Gap for MDI
People living with T1D who prefer multiple daily injections (MDI) of insulin now have two high-tech management options: An insulin…

Eli Lilly Acquires Breakthrough T1D T1D Fund Portfolio Company Protomer Technologies
Breakthrough T1D is excited to announce that the T1D Fund portfolio company Protomer Technologies, who is developing "smart" insulin, has been acquired by Eli…

Bigfoot Is Real—and Received FDA Clearance
The Bigfoot Unity™ Diabetes Management by Bigfoot Biomedical received FDA clearance for individuals 12 and older.
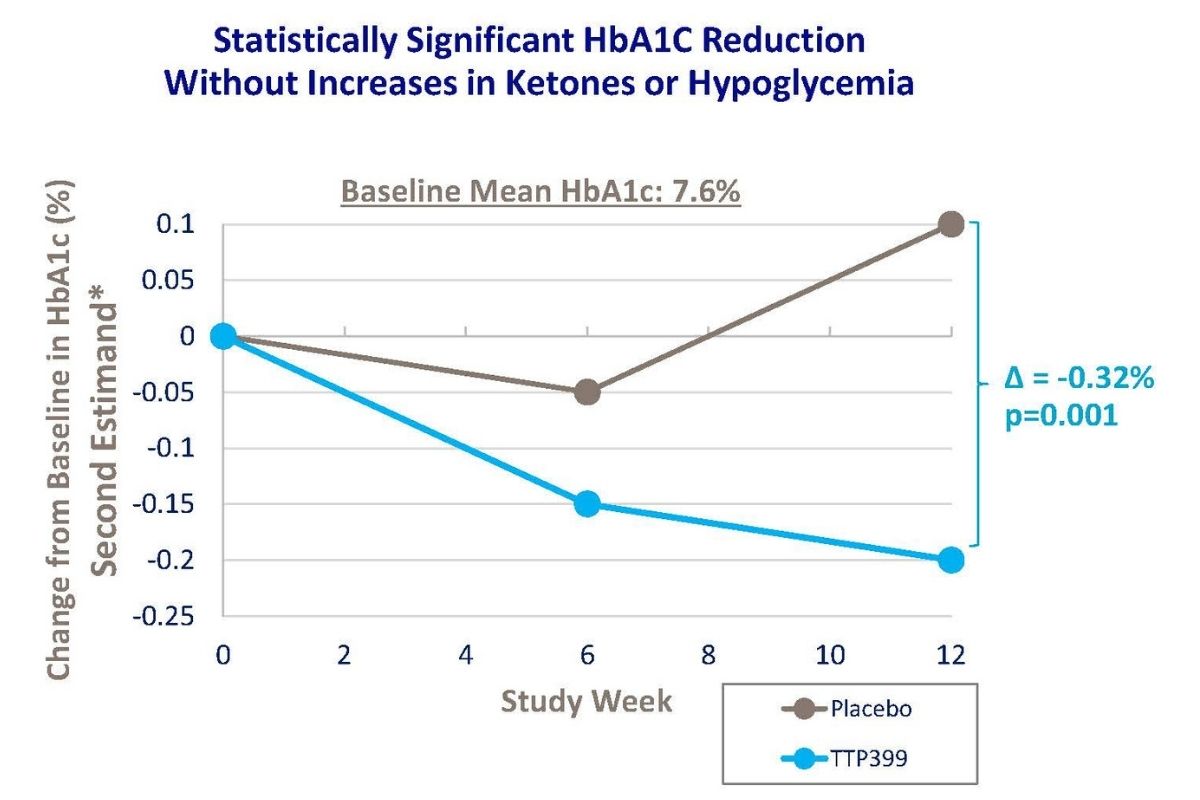
FDA Breakthrough Therapy Designation for TTP399—A Potential First-in-Class Adjunct Therapy in T1D
The FDA granted Breakthrough Therapy designation for vTv Therapeutics’ TTP399 as an adjunct therapy to insulin for T1D.

Severe Low Blood Sugar: There’s a New Treatment Option
The FDA approved Zegalogue® (dasiglucagon) injection, for the treatment of severe hypoglycemia in children and adults with diabetes aged 6…
