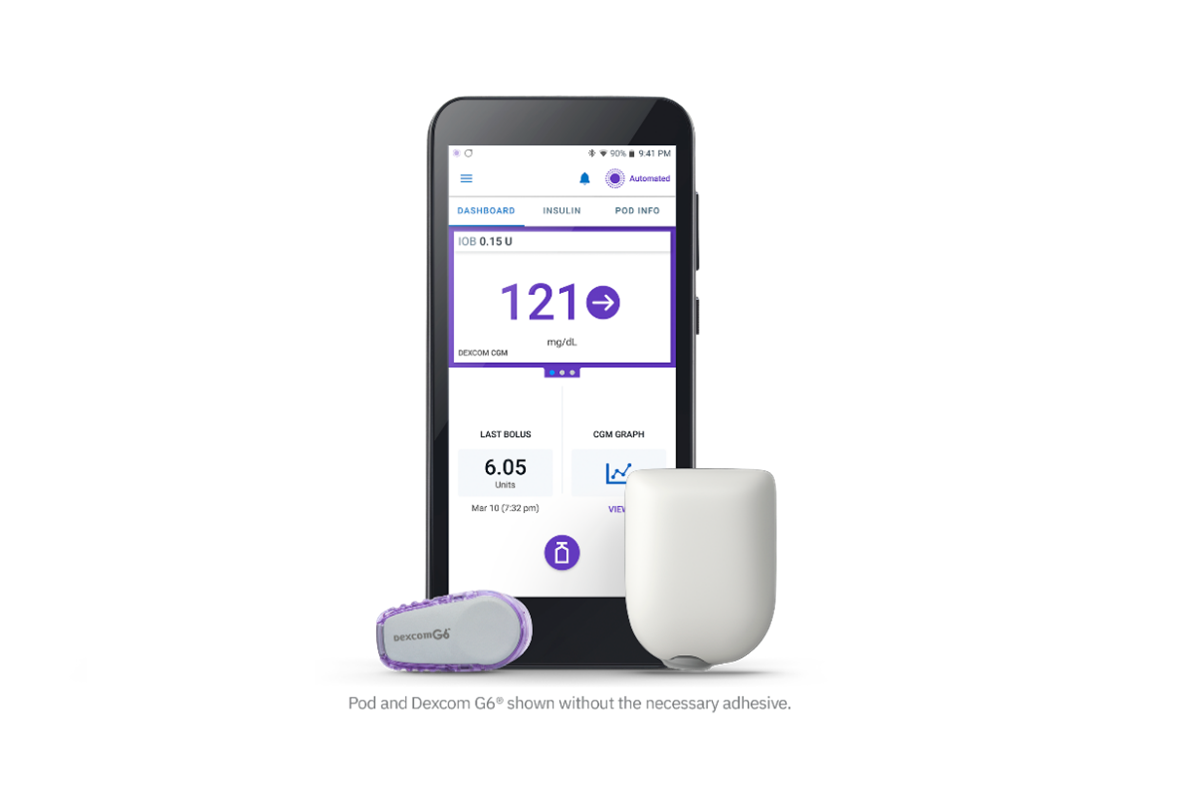
FDA Authorizes Omnipod 5 for Ages 2+ in Children with Type 1 Diabetes
The FDA authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system, for children 2+, using an algorithm that…

Tandem Diabetes Makes Managing T1D More Convenient
Tandem announced the FDA clearance of bolus insulin dosing from their t:connect® mobile app. Soon, individuals with the Tandem t:slim…

FDA Authorizes a Fourth Artificial Pancreas System
The FDA authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system for people 6 and older, using an…

New Study Shows Kids 1+ Benefit from Artificial Pancreas Technology
A new paper published in the New England Journal of Medicine shows hybrid closed loop systems help children as young…

Artificial Pancreas Systems: Sleeping Without Fear
Research shows Artificial Pancreas Systems can help improve diabetes-related outcomes and lighten the burden of T1D. Even during sleep.

FDA Approves 7-day Infusion Set
Medtronic’s Extended Wear Infusion Set (EWIS) is the first infusion set approved for 7 days, a significantly longer duration; Currently,…

SFC Fluidics, a Breakthrough T1D Partner, Receives Breakthrough Device Designation
SFC Fluidics, a Breakthrough T1D partner, received a breakthrough device designation for its interoperable insulin delivery pod. How is it different? Find out here.

FDA Approves an Artificial Pancreas System for Children Aged 2-6 Years
The Food and Drug Administration (FDA) approved the Medtronic MiniMed 770G artificial pancreas system for use by children aged 2…
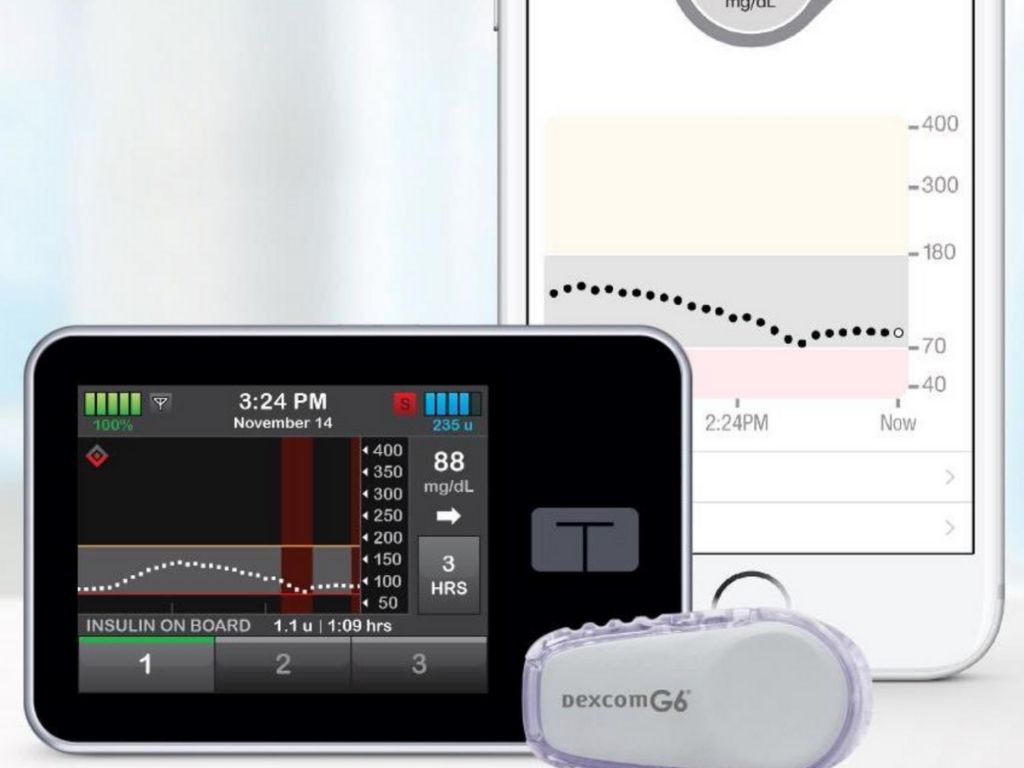
Tandem Control-IQ Controls T1D in Children Ages 6 and Up
A clinical trial at four pediatric diabetes centers in the United States has found that a new artificial pancreas system—which…
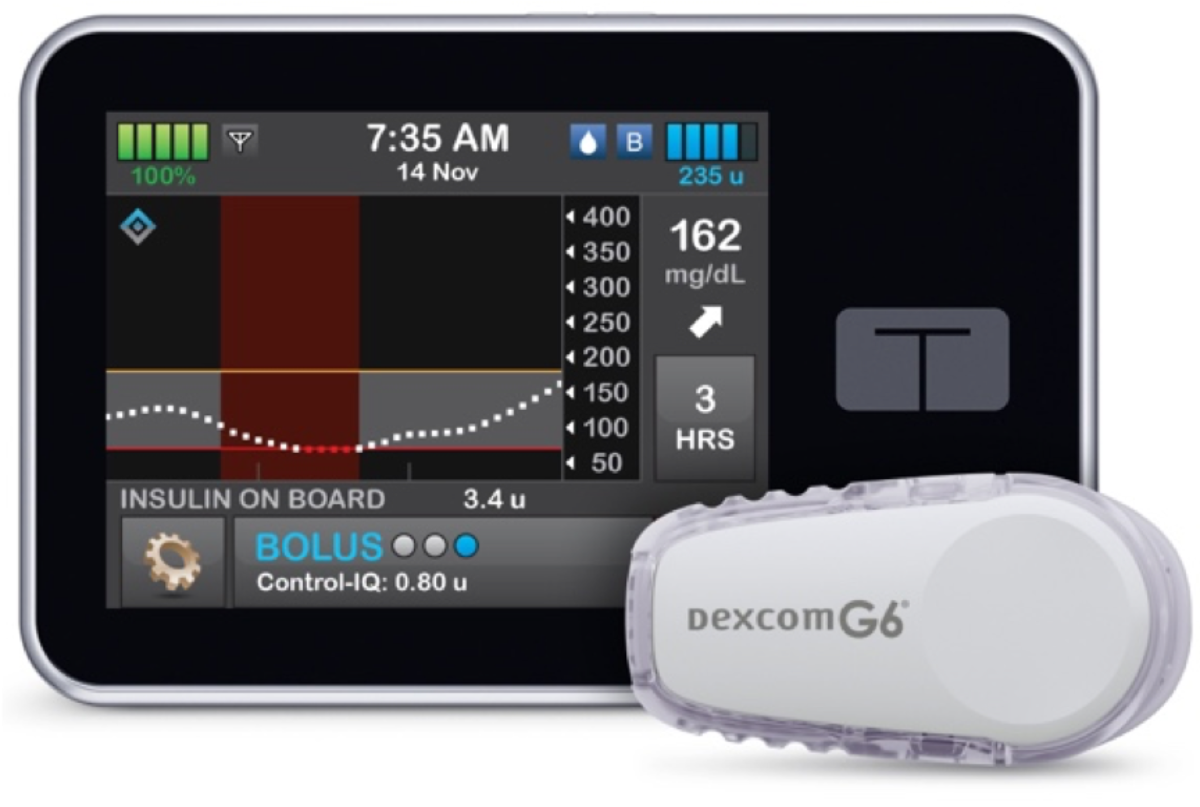
Tandem Control-IQ—Now Authorized For Children
Tandem Diabetes announced FDA clearance of an expanded pediatric indication for the Tandem t:slim X2™ insulin pump with Control-IQ™ technology…