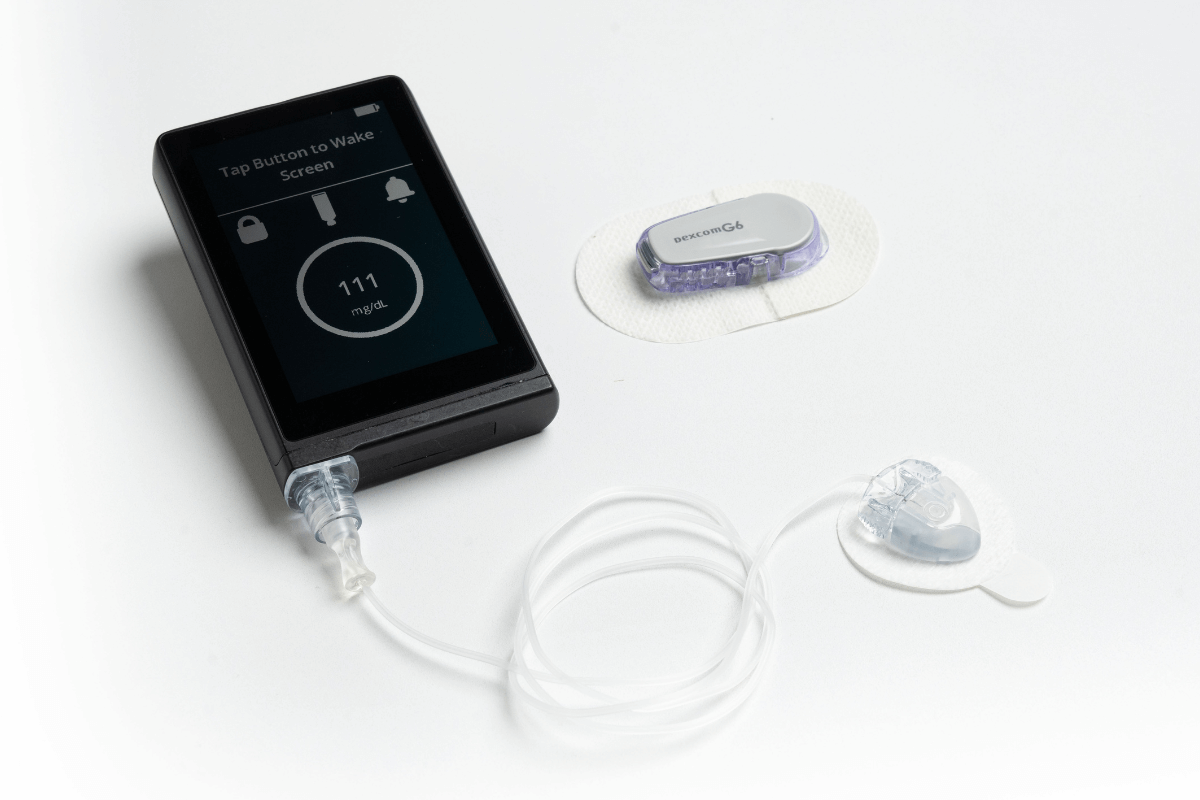
FDA Clears a New Artificial Pancreas System
The FDA cleared the iLet Insulin-Only Bionic Pancreas System for people 6 years of age and older with type 1…
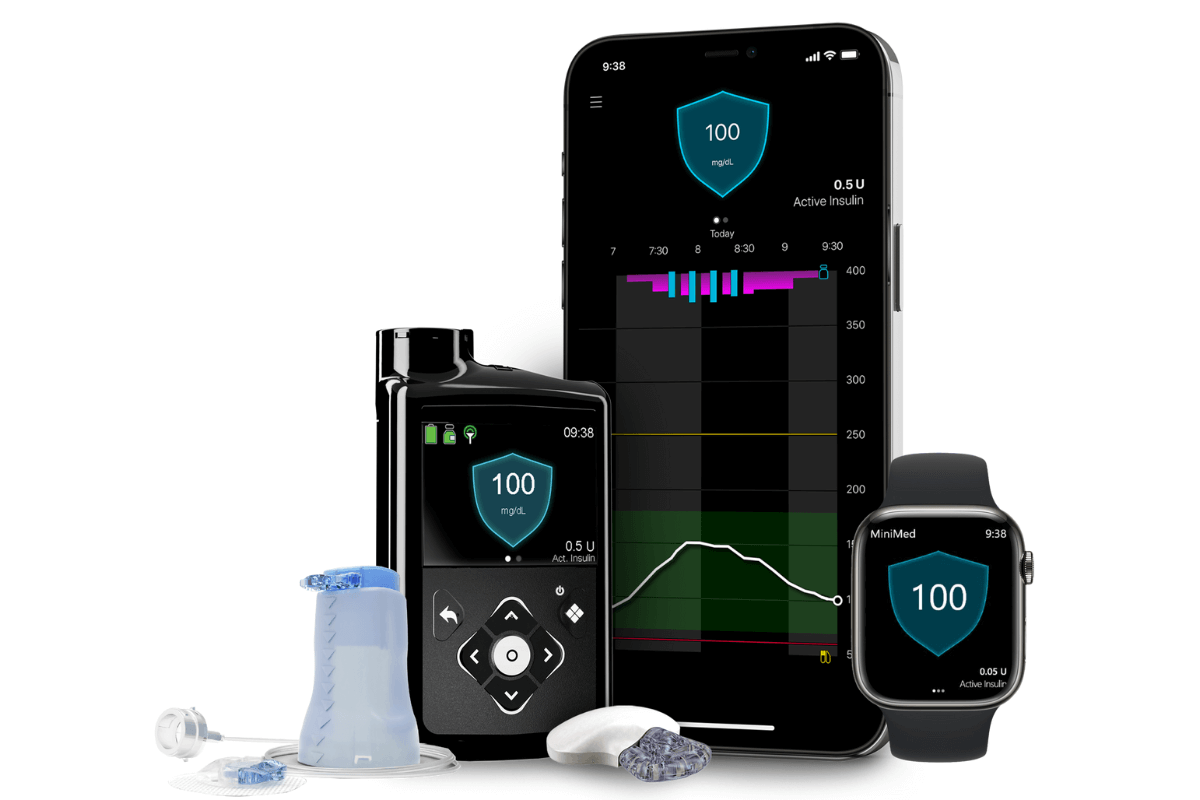
FDA Approves Medtronic 780G Artificial Pancreas System
FDA approved the Medtronic 780G artificial pancreas system, providing automatic adjustments and corrections to blood-sugar levels.

A Bittersweet Goodbye for Breakthrough T1D Champion Cynthia Rice
JDRF’s Chief Mission Strategy Officer will leave behind quite a legacy once she steps down from her role at Breakthrough…

FDA Authorizes Tidepool Loop, an Automated Insulin Dosing App
The FDA authorized the Tidepool Loop, an algorithm that could be used to work with commercially available insulin pumps and…

What We Can Be Proud of in 2022
There is tremendous progress in accelerating cures, improving lives, and advocating for people with T1D and their loved ones over…

Dexcom G7 Continuous Glucose Monitor Cleared by the FDA
Dexcom G7® Continuous Glucose Monitor system was cleared by the FDA, giving people with diabetes more options for managing their…

FDA Approves Tzield™—A Watershed Moment for the T1D Community
Breakthrough T1D celebrates the FDA approval of Tzield, the first disease-modifying therapy to delay clinical T1D in people at risk…
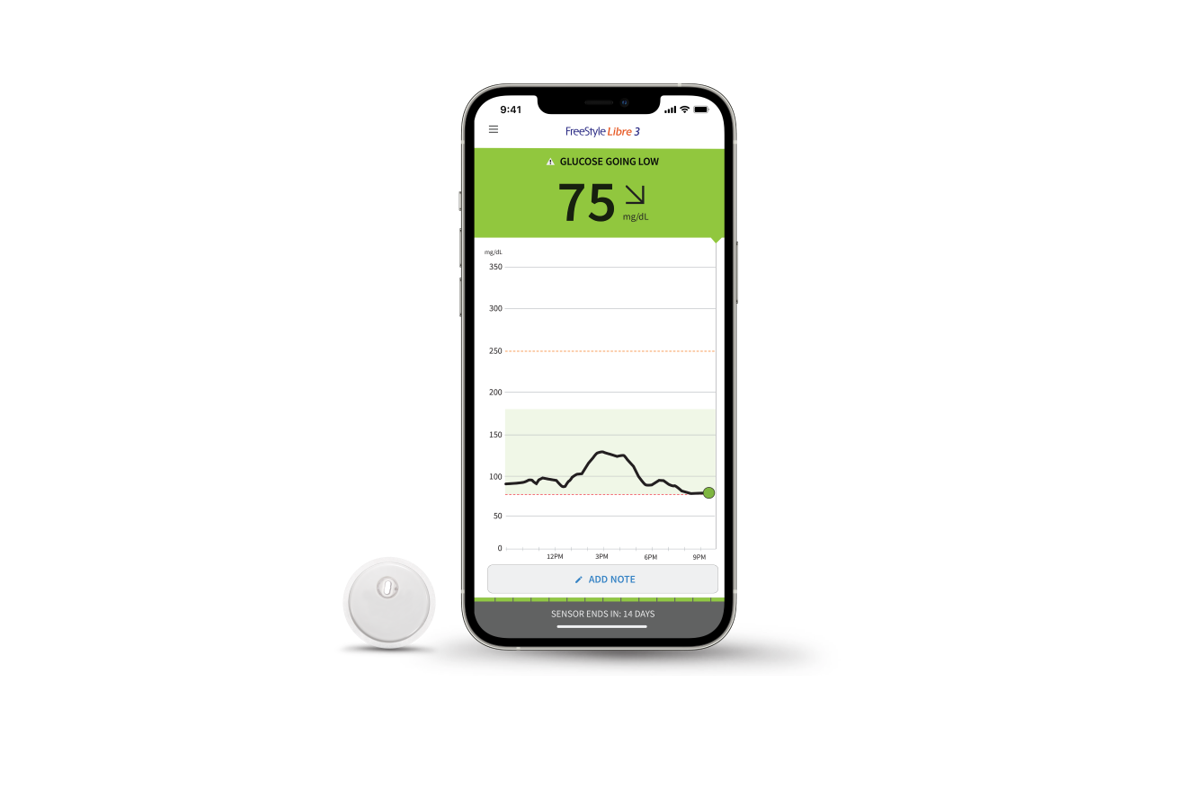
FDA Approved! Abbott FreeStyle Libre® 3 Continuous Glucose Monitor, Ages 4+
The FDA cleared the FreeStyle Libre 3 continuous glucose monitor (CGM) for children and adults ages 4 and up with diabetes

Advocacy in Action: Breakthrough T1D 2019 Government Day
After nearly 500 meetings with Members of Congress, Breakthrough T1D 2019 Government Day has officially come to a close. Over…

Eli Lilly Announces Lower-Cost Generic Insulin
Eli Lilly and Company announced it will begin selling a generic version of its most commonly prescribed insulin -- Humalog