
When Tzield was approved by the United States Food and Drug Administration (FDA), the type 1 diabetes (T1D) community had real cause to celebrate: The first disease-modifying therapy for T1D had cleared one of the last major hurdles to becoming available.
Disease-modifying therapies
Also "DMTs" for short, these therapies prevent, slow, halt, or reverse T1D progression.
But once Tzield was on the market and covered by health insurance companies and other payers, a new hurdle emerged: a majority of healthcare providers across the country were unaware of the drug, let alone how to administer it.
The clinical guideline for Tzield infusion did not become available until a year and a half after the FDA approved the drug. To date, 500 people in the U.S. with early stage T1D have received Tzield. Compare that to the annual incidence rate of T1D in the U.S. according to the T1D Index:
According to a 2023 study in the journal Diabetes Technology & Therapeutics, Tzield isn’t the only advanced T1D therapy with a surprisingly low adoption rate.
The FDA approved the first artificial pancreas (AP) system in 2016. Less than a decade later, there are now eight such approved systems on the market. These systems—also called automated insulin delivery (AID) systems—lead to better T1D management and health outcomes—yet only 16 percent of people with T1D in the United States use them.
Similarly, the FDA approved Lantidra, the first donor-derived cell therapy for T1D, in 2023. To date, one person has received it.
Increasing adoption to improve health
Closing the gap between access to and adoption of T1D therapies is a mission priority for Breakthrough T1D.
“It’s similar to the question: ‘If a tree falls in the forest and no one is there, does it make a sound?’” said Anastasia Albanese-O’Neill, Ph.D., APRN, CDCES, Associate Vice President of Breakthrough T1D’s Community Screening and Clinical Trial Education programs. “In this case, if you have a cutting-edge new therapy but most healthcare providers don’t know about it, don’t prescribe it, and don’t know how to administer it, does it have an impact?”
The organization recently announced the establishment of a Medical Affairs unit. The team will address the numerous challenges contributing to the slow adoption of groundbreaking T1D therapies, delaying their life-changing potential for many people living with the disease.
Challenges we are addressing:
HCPs have much greater knowledge of type 2 diabetes—or T2D—which is more prevalent.
HCPs need comprehensive guidelines to support new, approved treatment options.
There are too few clinical environments with the equipment and expertise to administer advanced T1D therapies and treatments, such as new T1D devices, therapies that require infusions like Tzield, and treatments that require implantation, such as cell therapies.
There are too few endocrinologists and certified diabetes care and education specialists with knowledge and competency in advanced T1D therapies.

With the establishment of our Medical Affairs team, we are reaffirming our organization’s commitment to creating a world where every individual with type 1 diabetes has access to life-changing therapies. By addressing systemic barriers and fostering clinical readiness, Breakthrough T1D will be pivotal in driving the timely adoption of emerging therapies and transforming care.”
As part of this organizational change, the Community Screening and Clinical Trial Education team, led by Albanese-O’Neill, will be integrated into Medical Affairs.
The team will focus on developing education materials for healthcare professionals in the U.S. and around the world; empowering people with T1D to participate in shared decision-making with their healthcare teams about emerging T1D therapies; helping to establish and socialize clinical care guidelines tailored to regional needs; and expanding clinical trial participation through community activation and HCP education.
“We have been doing a great deal of work to expand our HCP education, T1D community screening, and clinical trial education programs for more than three years now,” said Albanese-O’Neill, who has been with Breakthrough T1D as a fulltime staff member since 2022. “Given what we are seeing with adoption rates and with Dr. Danne joining us, we are now putting all of this work together in one department with a more strategic approach.”

Empowering clinicians with education
The team recently launched comprehensive, expertly redesigned HCP education and training resources.
These resources—which are accredited, free-of-charge, and live or on-demand—offer a significant focus on early detection for the earliest stages of T1D, monitoring guidance for positive test results, clinical trial opportunities, and the latest on cutting edge T1D therapy research and development, including disease-modifying therapies and islet cell therapies.
While designed specifically for healthcare professionals who can earn 4.5 credit hours of continuing medical education, the resources are available to the public. The on-demand feature means busy healthcare professionals with schedules that include all kinds of shifts imaginable can access this turn-key resource on their own time.
For a deeper dive, Breakthrough T1D’s resources will also offer live sessions, allowing time to interact with and learn from leading experts in the T1D field, including Albanese-O’Neill and Danne, in addition to those affiliated with different clinical facilities and institutions across the nation.

Our goal is to make this education as accessible as possible.”
Detecting T1D before symptoms present
A simple blood test can detect T1D in the earlier stages—before obvious symptoms develop. The biggest challenge is educating clinicians and the general population about it.
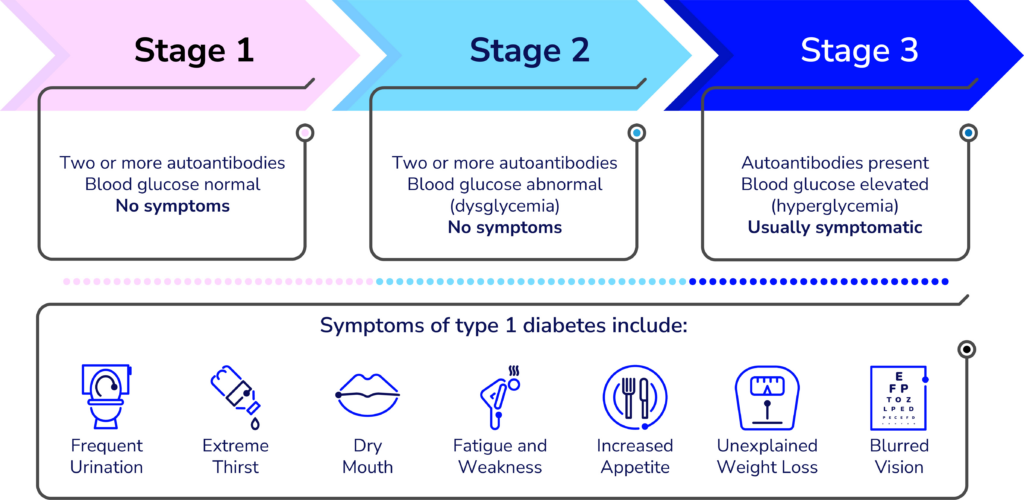
“Endocrinologists, Pediatricians, and some other specialty physicians learn about T1D screening and monitoring during their residencies, but it’s not a part of the general curriculum of the first four years of medical school,” said Lally, who built the learning management system for the new resources and is organizing the virtual offerings. “We’re working to advance that knowledge to yes, doctors, but also other clinicians whose patients could benefit.”
Many clinicians hesitate to order unfamiliar tests—especially if they are unsure what to do with the results. Most people who see any kind of healthcare provider could benefit from screening for T1D—according to a paper published in the journal US Endocrinology, roughly 85 percent of people diagnosed with type 1 do not have a blood relative with the autoimmune disease.
“Clinicians need to learn about the stages of type 1 and the specific autoantibody tests that identify type 1 versus type 2 and identifying type 1 in individuals at risk before they need insulin,” said Colleen Buggs-Saxton, M.D. Ph.D.
Buggs-Saxton, a Pediatric Endocrinologist at Wayne Pediatrics in Michigan, is the clinical leader of a Breakthrough T1D Early Detection pilot clinic. Using the new resources, she and Albanese-O’Neill are going to lead a grand rounds about T1D early detection at her institution, which is affiliated with the Wayne State University School of Medicine and healthcare system.

Clinicians should consider autoantibody testing for adults who have been diagnosed with type 2 but don’t have typical clinical features and require insulin to manage their blood sugars.”
“This is a novel way these resources can be used—as the basis of a locally and or virtually-provided grand rounds,” said Albanese-O’Neill.
While much of the emphasis of T1D early detection programs has been on children and teens, its applications are much broader—anyone can develop T1D at any age and unfortunately, misdiagnoses happen. According to an article published in the journal The Lancet, Regional Health: Europe, it is estimated that nearly 40 percent of adults older than age 30 with T1D may have been misdiagnosed with T2D.
“Most clinicians are very comfortable ordering an HbA1c test to classify people with type 2 diabetes, but they do not know what tests to order to classify people with type 1,” added Buggs-Saxton.
Grand rounds
Grand rounds are educational meetings and presentations for clinical teams at a given institution or healthcare facility to provide a summary of updates to the standards of care.

What to do with positive test results
The screening test is just the first part of T1D early detection. Clinicians also need to know what to do with positive results once they come in. Breakthrough T1D’s HCP resources offer extensive education on the topic.
Less than one year ago, Breakthrough T1D and other leading diabetes organizations developed monitoring guidance to help clinicians support people who test positive for stage 1 or stage 2 T1D. The guidelines have been endorsed by leading medical journals and organizations around the world.
”This monitoring guidance can help any clinician feel confident in providing adequate care in the early stages of type 1 and know when to refer to a specialist,” said Albanese-O’Neill.

I think of research, advocacy, and medical affairs as three legs of a stool—in terms of clinical adoption, each helps answer a different question. Research: Does it work? Advocacy: Will the regulators approve it and will insurance companies cover it? Medical Affairs: Do clinical teams have the competency and readiness to prescribe the treatment and educate and support people with T1D?”
It’s also helpful for the people who test positive for early stage T1D. Using the monitoring guidance, people can work with their healthcare team to monitor blood glucose levels to identify when insulin therapy may be needed; consider participating in clinical trials of disease-modifying therapies in development; and consider when and whether Tzield might be appropriate.
“There is currently one FDA-approved disease-modifying therapy for early-stage type 1 diabetes and additional therapies are being studied in clinical trials,” said Lally. “Identifying type 1 early gives the individual and their family time to learn more about type 1 and their options before reaching stage 3 T1D, which requires daily insulin therapy.”

Clinical trials: Increasing patient referrals
Clinical trials are a vital step for any treatment, drug, or device to make it into the hands of people with T1D. Currently, more than 300 clinical trials focused on T1D are actively recruiting participants.
Moreover, clinical trials can offer people the chance of receiving a cutting-edge treatment they may not otherwise be able to access.
Through its HCP resources and existing clinical trial resources, Breakthrough T1D is stressing the significance of investigational T1D therapies—while also clarifying common misconceptions about clinical trials.
“The clinical trial education portion of the program explains current trial opportunities and the critical need to increase diversity in diabetes research,” said Lally.
Despite the importance of clinical trials, many are delayed due to slow enrollment, adding cost and prolonging the results. A 2020 Tufts University study found that nearly 90 percent of clinicians surveyed felt comfortable talking about clinical trials.
“Unfortunately, the survey also revealed that annually, fewer than one percent of patients are referred to clinical trials,” said Lally.
So, why aren’t more clinicians referring their patients to clinical trials? Time and resources.

The most challenging part is helping patients understand what a clinical trial is, what it involves, and how previous scientific advances were only possible because of clinical trials. Healthcare providers often don’t have enough time in their busy clinics to discuss this with patients and families.”
Jacobsen, who is with University of Florida Health (UF Health), is one of the faculty members for Breakthrough T1D’s new HCP resources—specifically, the offering related to currently recruiting clinical trials for T1D disease-modifying therapies.
Jacobsen stresses that families and individuals with T1D also need specific education on the potential of receiving a placebo during a clinical trial—and why it’s an impactful part of any clinical trial.
Clinicians also may not know how to quickly get and stay up to date on current trial opportunities and how to get individuals who test positive for T1D autoantibodies involved.
“We provide a streamlined presentation about how to talk to families of people with T1D or people at risk for T1D about clinical trials,” explains Jacobsen, “We can direct families to one of several websites for more detailed information, like Breakthrough T1D’s Clinical Trial Finder.”

Cell therapies: Cures within reach
Cell therapies are one of the most promising approaches to curing T1D and one of the cornerstones of Breakthrough T1D’s cures research portfolio.
Advances in cell therapies have ramped up in recent years: participants in clinical trials of these therapies have been able to stop taking insulin. To speed this progress even more, Breakthrough T1D recently launched Project ACT (Accelerate Cell Therapies).
Cell Therapies
Also called islet cell therapies, these therapies replace destroyed beta cells so that people with T1D can again produce their own insulin.
The organization has long invested in cell therapy research and has a track record of success in making life-changing T1D therapies a reality—the prime examples being artificial pancreas systems (AP systems) and Tzield.
The work of Breakthrough T1D’s Research, Advocacy, and Medical Affairs teams—in partnership with the organization’s venture philanthropy fund, the T1D Fund—will be integral to Project ACT’s success.
Like AP systems, Tzield, and all other FDA-approved drugs, treatments, and medical devices on the market today, islet cell therapies will only become available after meeting all the required benchmarks—including clinical trials.
Clinicians who are in-the-know about clinical trials and how to help their patients enroll are but one of the numerous ways Breakthrough T1D’s Project ACT will make islet cell therapies a reality, faster.
“Clinicians are generally the most trusted source for this information, but most are not making those referrals, so the gap never closes,” said Lally. “We aim to change that.”
“We want every member of the diverse T1D community to be aware of clinical trials, how to participate, and where to find information,” added Albanese-O’Neill. “The next generation of breakthroughs depends on it.”
Editor’s note: This story co-written by Ginger Vieira, special contributor to Breakthrough T1D.