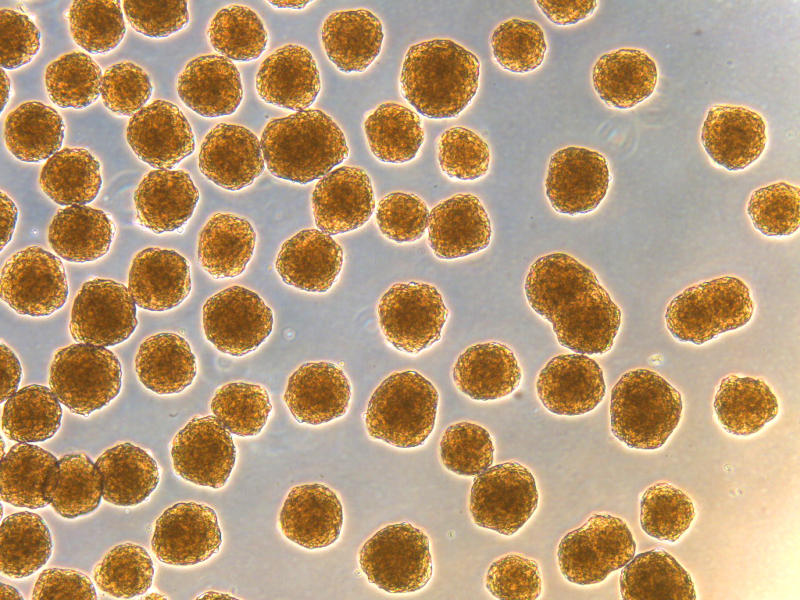
Jeffrey Millman, Ph.D., is a powerhouse of diabetes research. He was the co-author of a publication that outlined the strategy for generating insulin-producing (beta) cells from stem cells, in 2014. Since then, he has made several refinements, and published, in 2019 and 2020, updated strategies for generating highly functional insulin-secreting islet cells in the lab—which are the closest beta cells yet, to resemble human islets—from stem cells. This past year, the Millman Lab has succeeded in genetically engineering patient cells to become nondiabetic and reverse diabetes in mice, emphasizing the potential for personalized medicine.
Thus far, however, making these high-quality islets in the laboratory is a manual process, requiring investigators to follow a rather complex and meticulous multi-step process day after day. And it takes time and money. The cells have to go through 6 distinct stages over a month. Moreover, these cells are in high demand by the scientific community, as they are very valuable to accelerate research and enable researchers to focus on developing solutions to overcome other barriers and challenges preventing the widespread application of beta cell replacement therapy.
So, is there a way to fully automate the process of making islet cells, such as an automated production line? The answer is yes, but Dr. Millman and Breakthrough T1D needed a partner that could make it possible.
Automated Fabrication of Islets
The obvious partner was ARMI. It stands for Advanced Regenerative Manufacturing Institute. Their mission: to make practical the large-scale manufacturing of engineered cells, tissues and organs—like the lung, liver or even stem cell-derived pancreatic islets—via automation.
With Breakthrough T1D support, ARMI is translating the Millman procedure into a robust, reproducible and automated process to generate an unlimited amount of stem cell-derived human islets. The goal is to enable the production and distribution of sufficient numbers of quality-controlled islets to the scientific community for research purposes and eventual development as a replacement therapy for the treatment of people with T1D. Um, cool!
Filling a Gap in Cell Replacement Research
Now, why is Breakthrough T1D funding cell manufacturing for research purposes? Well, there is a gap between the cell manufactures for clinical trials and focused on making beta cell replacement therapies for T1D a reality, and the scientific community that needs a “best-in-class” source of islets to develop technology on how to protect such cells from the immune system after transplantation. Think of engineers developing implantable scaffolds or encapsulation devices, or an immunologist developing hydrogels with immunomodulatory properties. That’s where Breakthrough T1D steps in, to make this vision—bringing the Millman team’s expertise in stem cell technology and ARMI’s expertise in automation—into a reality.
Dr. Millman has received a Career Development Award from Breakthrough T1D, which provides 5 years of support to help him transition to a career in biomedical science and position himself at the leading edge of T1D research. Learn more about the researchers we fund, and learn how you can support their work to prevent, treat and—one day—find cures for T1D.