At Breakthrough T1D, our vision is to make our clinical trials more efficient (trial design optimization for quality, speed and cost) and effective (trial design optimization for approval, access and adoption). That’s why, in May 2019, Breakthrough T1D and The Leona M. and Harry B. Helmsley Charitable Trust held a two-day Innovative Trial Design Workshop, with the objectives to:
- Gain an understanding about types of innovative trial designs and paradigms, why and when to use them and identify barriers to making therapy development more efficient and less costly
- Provide an opportunity to derive lessons from clinical trials in other diseases
- Understand the strength and challenges to innovative trial design participation and define next steps for a Breakthrough T1D implementation plan
Why Now
There are many challenges in therapy development for type 1 diabetes (T1D), including disease heterogeneity and lengthy trial endpoints, and high attrition rates of clinical trials poses an economically insurmountable burden. There are modern tools and technologies—including Electronic Health Records, registries and Artificial Intelligence (AI) tools—that could help advance the research and development (R&D) process, to ultimately bring more devices and treatments to clinical trials and choices of approved therapies to the T1D community.
What
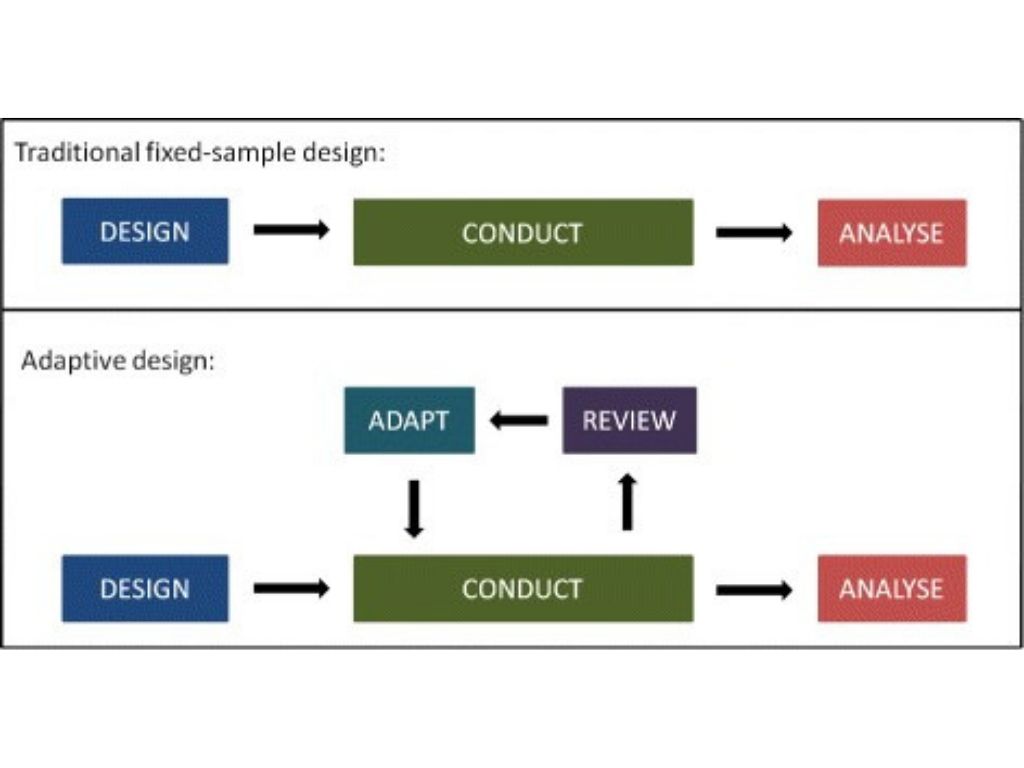
Breakthrough T1D and the Helmsley Charitable Trust brought stakeholders together from industry and academia, to listen and learn what these innovative tools were and discuss next steps for Breakthrough T1D. T1D experts shared their experience with T1D disease heterogeneity and clinical trial design challenges. Innovative trial design experts shared what the different innovative trial design tools are and how and when to implement them. The meeting also included breakout sessions where each team was asked to re-design a standard design trial with an innovative tool. The workshop was co-chaired by Roger J. Lewis, M.D., Ph.D., of Berry Consultants, a group that specializes in innovative trial design, and Colin Dayan, M.D., Ph.D., of Cardiff University.
Innovative trial designs come with their unique set of benefits and complexities. The benefits are:
- Matching the right treatment to the right patient to improve the probability of success for the patient
- To address the heterogeneity of the disease and the interplay with the molecular make-up of the patient
- Efficiently measure the treatment response and strategically help inform meaningful outcomes to a trial
But this requires skilled personnel, tools and infrastructure and a clear methodology to engage key stakeholders at different stages, i.e., regulators, pharma, industry, academia, payers, etc. Still, the advantages of these innovations are beneficial to everyone involved: patients, investigators, sites, sponsors.
Next Steps
Over the next few months, Breakthrough T1D and the Helmsley Charitable Trust will develop next steps for the implementation of clinical trial design enhancements, including a white paper or policy document coming out before the years end.
| T1D Experts | Innovative Trial Design Experts | People with Diabetes |
| Colin Dayan, M.D., Ph.D. (Co-Chair), Chair, Clinical Diabetes and Metabolism, Cardiff University | Roger J. Lewis, M.D., Ph.D. (Co-Chair), Chair, Department of Emergency Medicine, Harbor-UCLA Medical Center / Senior Medical Scientist, Berry Consultants | Randy Anderson, Ph.D. |
| Roy Beck, M.D., Ph.D., Executive Director, Jaeb Center for Health Research | Michelle Detry, Ph.D., Director of Adaptive Trial Execution and Senior Statistical Scientist, Berry Consultants | Devon Jones |
| Carmella Evans-Molina, M.D., Ph.D., Associate Director, Indiana University for Diabetes and Metabolic Diseases | Lisa LaVange, Ph.D., Professor and Associate Chair, Department of Biostatistics, UNC Chapel Hill | Mariel Lechner |
| Michael Haller, M.D., Pediatric Endocrinology Chief and Fellowship Director, University of Florida | Victoria Manax Rutson, M.B., BCh, B.A.O., Chief Medical Officer, Pancreatic Cancer Action Network | Margery Perry |
| Peter Senior, Ph.D., Medical Director, Clinical Islet Transplant Program, University of Alberta | Adrian Mander, Ph.D., Director of Statistics, Clinical Trials Research Unit, Cardiff University | Jen Schneider |
| Karen Lynn Price, Ph.D., Senior Research Advisor, Eli Lilly and Company |
Image: BMC Medicine 16: 29 (2018)