A protein, called glucokinase (or GK), acts as a key regulator of sugar levels in the body. If blood glucose levels are deemed too high, activation of GK in the liver causes the body to use more glucose, which in turn lowers glucose levels in the blood. vTv Therapeutics has developed a GK activator, called TTP399. The company joined forces with Breakthrough T1D in 2017, to test TTP399 in people with type 1 diabetes (T1D). The results of the first phase of it are out, presented at the 55th Annual Meeting of the European Association for the Study of Diabetes (EASD).
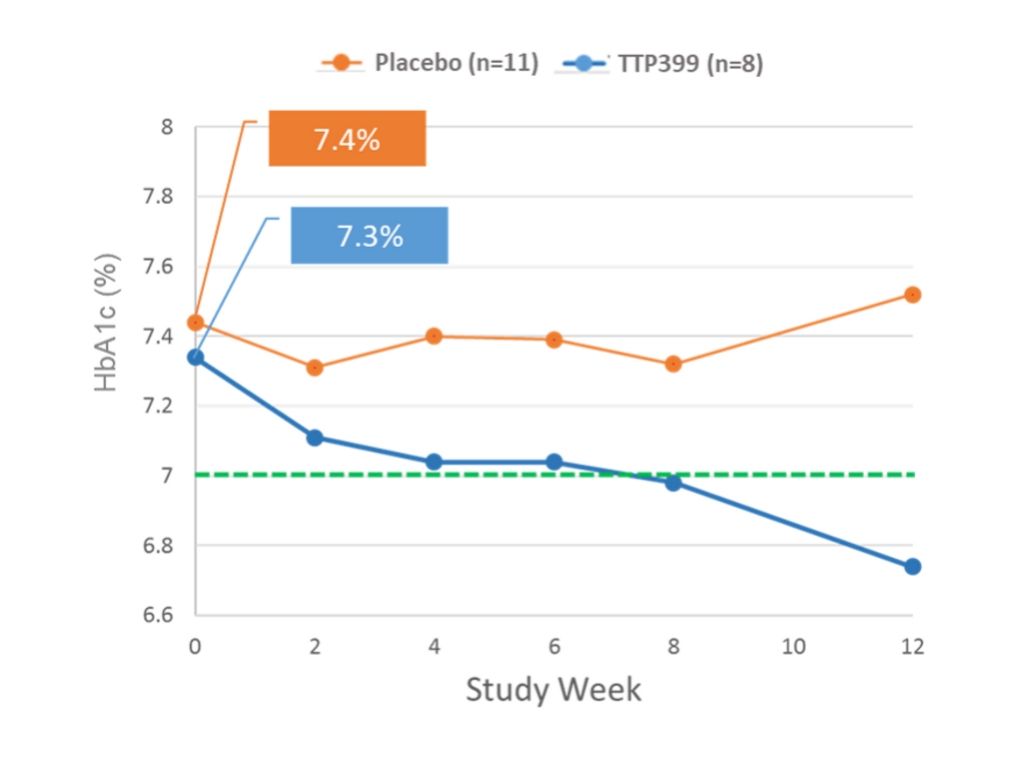
Nineteen people were recruited for the Breakthrough T1D clinical trial—eight to the group getting TTP399, and 11 to placebo. At 12 weeks, the TTP399 group had a significant and clinically meaningful reduction of 0.6 percent in their HbA1c, whereas those in the placebo group increased their HbA1c by 0.1 percent. At the same time, the group treated with TTP399 showed a trend of decreased insulin usage. There was no severe hypoglycemia and no DKA—both of which are complications of T1D—reported in either group.
These promising findings support advancing to phase II-part 2 to confirm the results in a larger and more diverse T1D population.