Getting in the holiday spirit: Fundraising to end T1D
Christmas light displays and cookies for a cure: Here's how some Breakthrough T1D community members are raising funds this holiday season.

Breakthrough T1D Ride 2025 Wrapped
You rode. We cheered you on. Together, we fueled type 1 diabetes breakthroughs! Read our wrap-up of the Breakthrough T1D Ride 2025 season.

Q&A with San Diego Padres Pitcher Mason Miller
Breakthrough T1D sat down with Mason Miller to learn more about his journey with type 1 diabetes and the impact it has on him as an athlete.

My Cause, My Cleats: Lacing up to drive meaningful change
Breakthrough T1D is honored to have the support of nine NFL players, coaches, and staff for the 2025 My Cause, My Cleats initiative.

Hot off the press: Continuous ketone monitoring guidelines are here
What’s happening? Continuous ketone monitoring (CKM) is on its way. A new paper in The Lancet Diabetes & Endocrinology, titled “International expert recommendations on the application and utility of continuous ketone monitoring for people with diabetes,” was published by Breakthrough T1D and expert collaborators, spearheaded by our Chief Medical Officer, International, Thomas Danne, M.D., Ph.D. […]
Support and resources for your CGM
Support and resources for issues with continuous glucose monitors (CGMs), including inaccurate readings and faulty sensors.

2025 Top T1D advances: Full speed ahead
How quickly a year goes by—and a lot has happened for type 1 diabetes (T1D) in 2025! In these last few weeks leading up to the new year, Breakthrough T1D would like to take a moment to reflect on how far we’ve come in the past 365 days. Read on for the greatest T1D accomplishments […]

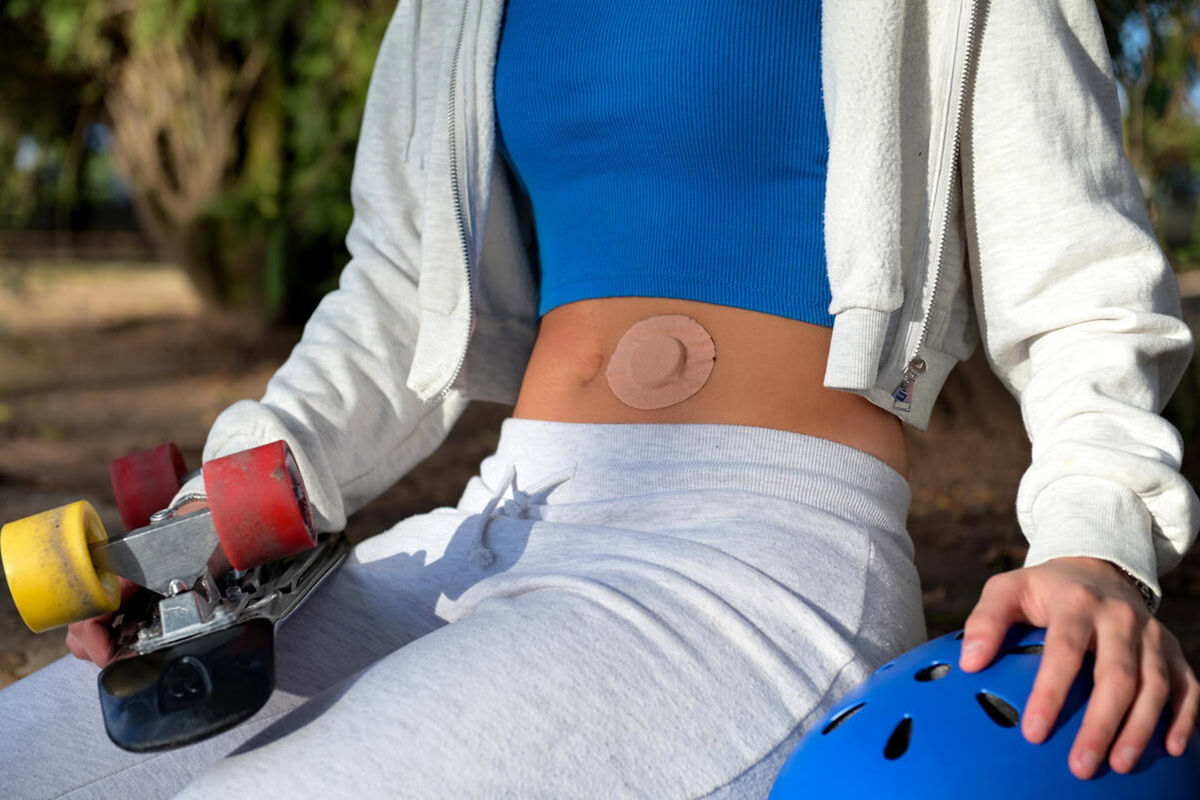
Smart devices, angry skin: The underside of diabetes tech
Skin reactions from adhesives on insulin pumps and CGMs are common. Learn why they occur and how to reduce irritation.
See Medical Affairs. See T1D.
Closing the gap between access to and adoption of type 1 diabetes therapies is a mission priority. Chris Dunn represents these efforts in action.

A week-long, intercontinental marathon journey for type 1 diabetes
Forrest Johnson embarked on a globe-spanning, 184-mile sprint with The Great World Race to fundraise for type 1 diabetes research.