The U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation for vTv Therapeutics’ TTP399 as an adjunct therapy to insulin for type 1 diabetes (T1D). Once granted, Breakthrough Therapy designation is intended to expedite the development and review of drugs for serious and life-threatening conditions.
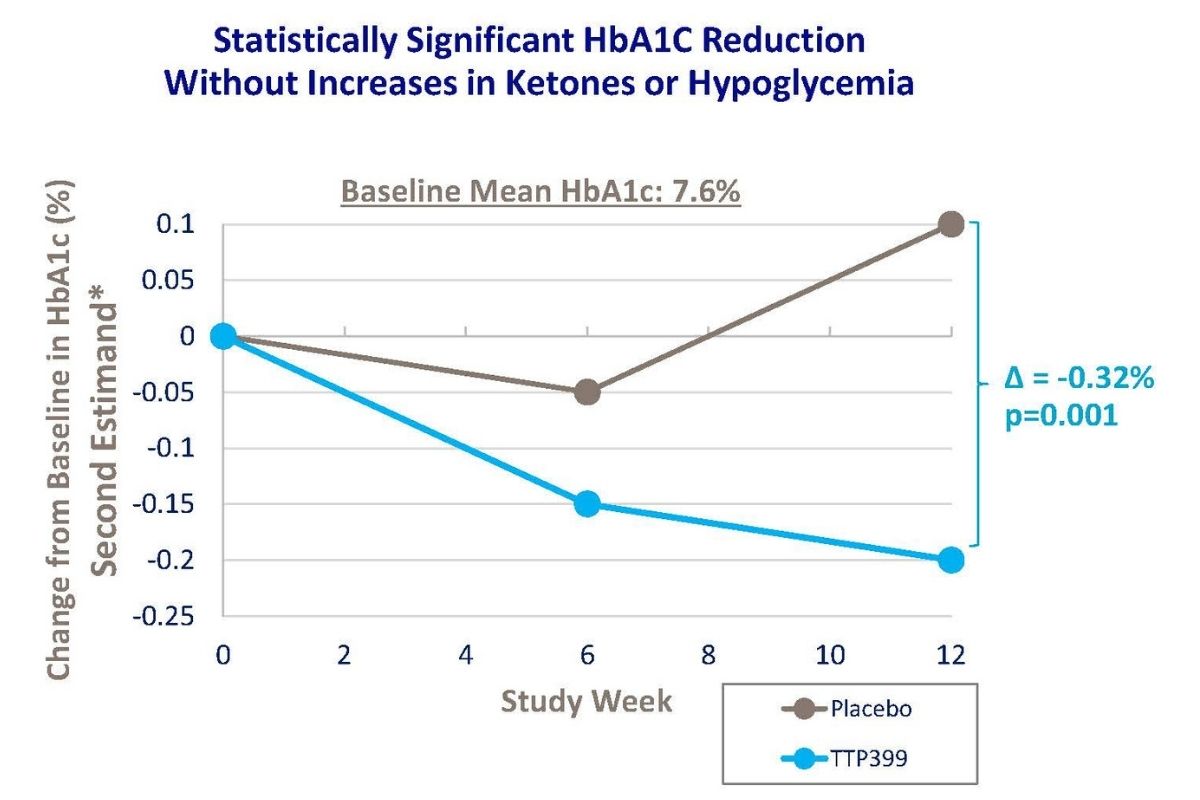
In a JDRF-funded phase II clinical trial called Simplici-T1, TTP399 significantly improved HbA1c in people with T1D. Additionally, trial participants who received TTP399 showed a reduction in insulin dose, reduced hypoglycemia (low blood sugar), and no increase in diabetic ketoacidosis (DKA).
TTP399 is a liver-selective glucokinase (or GK) activator. GK acts as a key regulator of sugar levels in the body. When blood-sugar levels rise, activation of GK in the liver stimulates glucose utilization, which in turn lowers glucose levels in the blood.
After several human studies in type 2 diabetes, vTv Therapeutics joined forces with Breakthrough T1D in 2017, to test TTP399 in people with T1D. The positive topline results from this phase II clinical trial follow the positive results obtained in the previous smaller clinical study reported by Breakthrough T1D in June 2019.
The next step: Upcoming pivotal trials and a mechanistic study to test the effects of TTP399 on diabetic ketoacidosis (DKA).