
Challenges and solutions: The present and future of cell therapies for T1D
Breakthrough T1D is funding research that supports manufactured islet cell source, survival, and protection and is also driving initiatives to…

Breakthrough T1D 2025 Government Day: 4 amazing days, 485 Congressional meetings, 370 sore feet
Breakthrough T1D headed to Washington D.C. where advocates held nearly 500 meetings with Members of Congress on behalf of the…

2024 greatest T1D hits: A glance at this year’s achievements
While we look back on 2024, we can reflect upon the incredible progress we’ve made in advancing breakthroughs toward cures…

New ICD-10 Codes Will Help the T1D Community
On October 1st, the T1D community got a big win: the Centers for Medicare and Medicaid Servies (CMS) introduced a…

Advocating for progress and access
With the support of our grassroots advocates, our work helps advance treatments, influence policy, and improve access to care for…

Breaking through to Congress at 2024 Government Day
Our Advocates traveled to D.C. to reintroduce our organization and champion legislation and policy for the T1D community.
Beating the Odds: The Improbable Story of How Perseverance, Belief, and Luck Led to the Approval of the First Life-Changing Immune Therapy for Type 1 Diabetes
The true story of how Tzield (teplizumab), the first disease-modifying therapy approved for use in delaying type 1 diabetes (T1D)…

What Drug Will Be the Next Tzield?
In 2023 alone, there have been several important papers published on disease-modifying therapies (DMT) for type 1 diabetes. This blog…
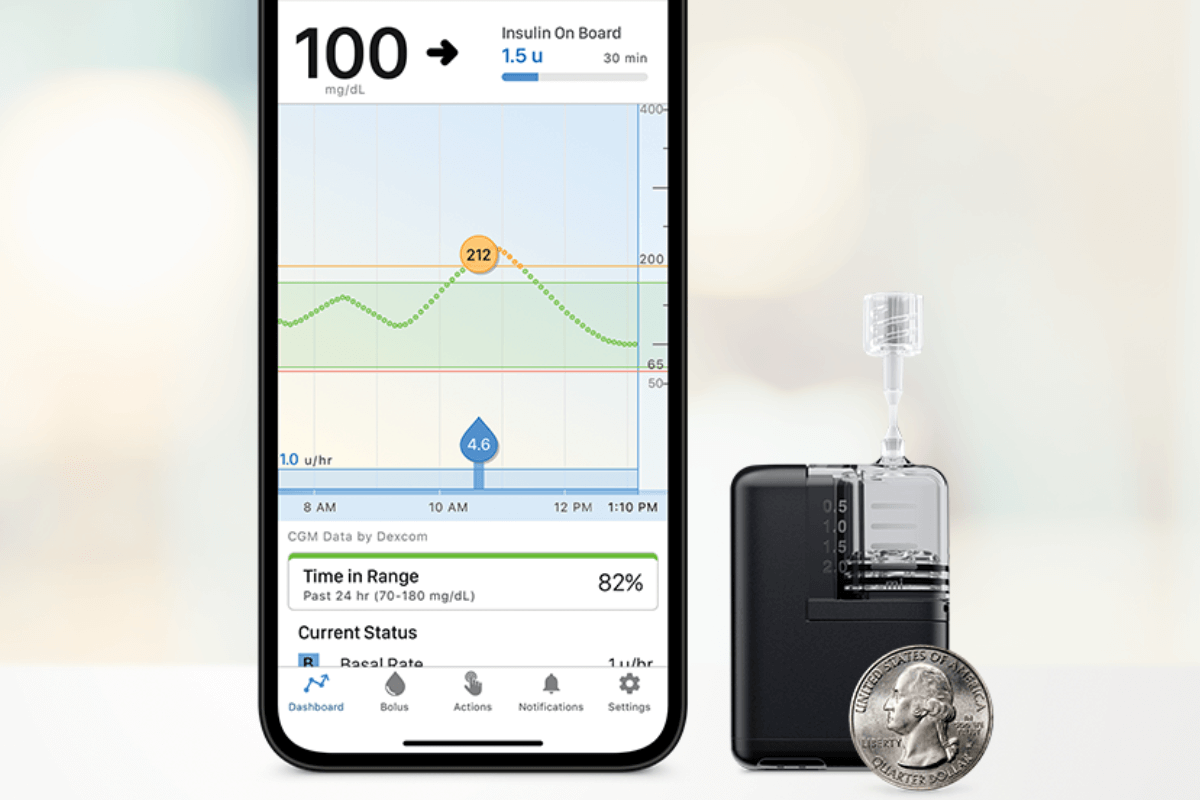
The Smallest Artificial Pancreas System Receives FDA Clearance
Tandem Mobi—a miniature-sized insulin pump, for use with Tandem’s Control-IQ™ technology and a compatible CGM—received FDA clearance.

Top Type 1 Diabetes Advances of Fiscal Year 2023
Breakthrough T1D highlights the top type 1 diabetes advances we've seen during fiscal year 2023, including cures and life-improving therapies.