This article is produced from the transcript of the Research Information Volunteer Talk from October that was presented by Dr. Sanjoy Dutta, Chief Scientific Officer, Breakthrough T1D. This is the link to this talk on the Breakthrough T1D YouTube Channel: https://youtu.be/dJzkvIZcbd0?si=1Q5anpk6IAaLip0F
Today the mission remains with the focus on improving the lives of everyone that has type 1 diabetes (T1D), by accelerating life changing breakthroughs that can cure, prevent, and treat T1D and its complications. Breakthrough T1D prioritizes the research funding that has the highest likelihood of accelerating our mission while maintaining strategic funding in areas that improve the lives of people with diabetes in the United States and around the world. Our work is more important now than ever.
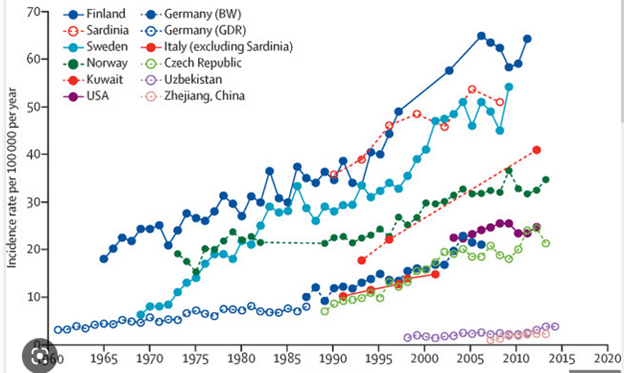
The reality today is that the incidence and the prevalence of T1D is increasing, unfortunately, all over the world, and it is increasing in different ethnic populations, certainly in all geographies and in all age groups. The problem is bigger than we knew when we were talking about it 20 years ago. The leadership role Breakthrough T1D plays is even more important now.
Breakthrough T1D works across the pipeline to get these therapies to the T1D community. Breakthrough T1D goes from the initial light bulb idea in someone’s head and the initial research to product development and regulatory approval. It is also important in getting the treatment covered and getting clinicians to understand the value proposed by the treatment and what stages can benefit from the treatment. This helps to ensure the adoption of the therapy. One of Breakthrough T1D’s top priorities is what we are doing to cure, as well as improve, the lives of those living with T1D. These are biological cures, often called disease modifying therapies. Research is working on how can we provide insulin independence and better glucose control while also working on new cell therapies. We need to find those who are at risk and improve screening of these individuals. Improving lives is a broad area that affects the 1.4 million Americans, and 8 or 9 million people around the world today who are living with T1D – and the numbers are going up.
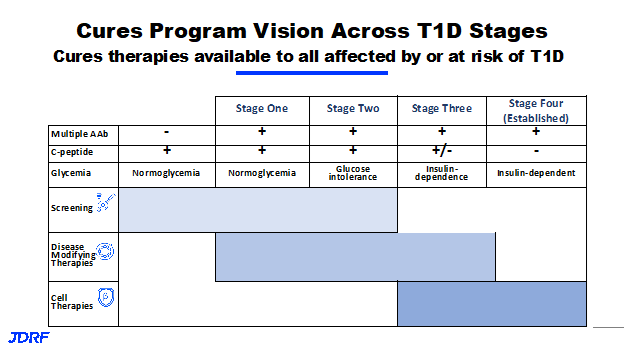
Disease modifying therapies is part of the Breakthrough T1D mission to cure T1D. There have been several versions of the table below that depicts the staging of T1D, showing progressive loss of insulin production.
This table shows ways of staging the people with T1D, starting with stage zero where people still have full insulin production and full glucose control. They do not have any signs of autoimmunity yet. They may or may not be at a genetic risk, and then the onset of T1D starts through stages 1 and 2, where there are increasing signs of autoantibodies in the blood, or the autoantibodies are active and beginning to destroy and cause instabilities in the beta cells. This is when the body slowly loses the insulin-producing capacity and loses the internal glycemic control that the pancreas provides. This is when a person enters into a stage three, and will need external insulin treatment to be able to control blood glucose levels. Today, that is when the clinical diagnosis of T1D happens. We now know very well that T1D actually starts years or decades before stage three or insulin dependence happens, and now we can stop the progression. Stage four is sometimes called established type one diabetes, where people are essentially completely insulin dependent. The various curative programs are in the color bars on the above table. Stay tuned for deeper dives on each topic throughout the year. There will be talks on each curative area in the coming months.
In disease modifying therapies, we are trying to address an active immune system that is destroying the body’s own pancreatic beta cells that makes insulin. We also need to find a way to thwart the immune system that is destroying the beta cells without affecting the normal immune system process. This is the balance that needs to be achieved. We need to be able to regrow beta cells to replace those that are destroyed and restore the insulin-producing capacity that has already been compromised. Regrowing the beta cells is sometimes called regeneration and in order to cure the disease, we will have to address both the problems in T1D. There has been a lot of progress, which can be seen on the slide below.
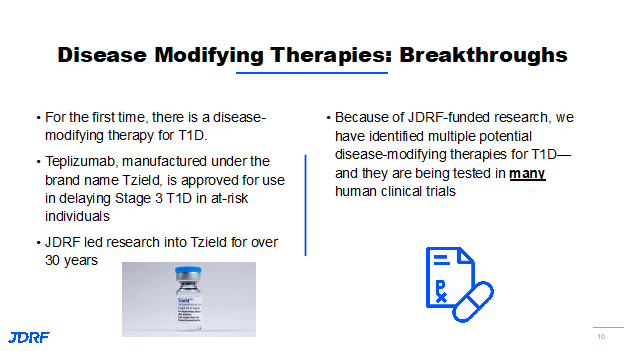
Last year, the FDA approved the first therapy to prevent T1D called TZield. This took decades of research to become available for those in stage two. TZield is recommended for individuals starting at age 8. Currently, there is a trial called PETITE-T1D for children under age 8. For TZield, individuals need the autoantibodies and an active immune system that has compromised insulin-producing ability, but do not require insulin yet. This trial is currently enrolling and interested individuals would need to know if they have the autoantibodies that lead to T1D before enrolling. This particular therapy delays the onset of insulin dependence by about three years. Studies are still underway to see if the effect can last longer.
There are some very promising therapies coming up in the next few months to the next few years. In order for disease modifying therapies to be successful and know who these therapies can work for, we need to have participation in clinical trials. We know that not all therapies are going to work for everyone, and not all therapies will work as well for everyone. Some people may benefit from two or more therapies. For this reason, we need to know who is at risk. Screening, as well as monitoring these individuals who have developed autoantibodies, is a very important priority.
T1D is on the rise in all geographies and in all ethnicities. This is a global problem and Breakthrough T1D is a global organization trying to address the rise in T1D. We get some important acute benefits from screening and monitoring: essentially eliminating a very dangerous condition called diabetic ketoacidosis (DKA), which can occur if individuals who are diagnosed have not been aware that they had autoantibodies that lead to T1D. A new diagnosis is much better if you can avoid being in DKA. Avoiding DKA has benefits that relate to several long-term outcomes, such as mental health, cognitive and behavioral health, complications, and better A1C. The A1C is still a key marker in predicting complications. There is a definite benefit if individuals can screen and monitor and avoid DKA.
The essential component is to identify these people, put them into clinical trials (providing that they are willing and able to participate) and accelerate the development of disease modifying therapies. This slide shows three areas of focus. We need to know how we monitor our efforts, what the screening tools are, what the monitoring tools are, and how can we build this evidentiary basis through research and through several efforts that can then be rolled out into community that can be broad scale. We do not need to be in a research setting or in a hospital setting within the United Stated or anywhere in the world; we can roll it out into the masses so that a pediatrician or general adult practitioner can be screening the people that come into their clinic. It can be rolled out to the practice of endocrinology as well. For this, we have the T1Detect program, which presently is in the United States. Other programs around the world are addressing community screening and monitoring, as well as critical components in education, education of policymakers, and so forth. That obviously leads into a huge policy effort, mostly in the health policy area. We want to ensure that while this research is building the body of evidence and while we are rolling out community screening, we are actually aiming for the north star, which is full coverage by the preventive service area for the screening of every individual in the general population, the way there are screenings for cholesterol and heart disease and high blood pressure and various antigen markers for certain cancers. We can make this routine care toward preventive services. That is our north star goal.
This slide shows various screening and monitoring programs that Breakthrough T1D is involved in around the world, and there are several others that Breakthrough T1D was not directly involved in here. Since this is a global problem, we have a robust effort in various parts of the world. Our goal is to get this data as evidence that can build consensus for policymakers around the world that screening and monitoring for T1D should be covered and available.
We can identify these people early on so they can participate in clinical trials and receive benefits from the therapies. Now that TZield is on the market, they can actually predict the odds of delaying the onset of diabetes. For the newly diagnosed individuals, TZield may extend the period in which the beta cells are still healthy and functioning.
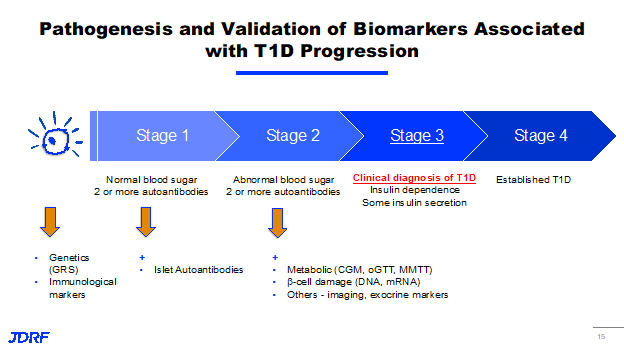
This slide shows the linear progression of the disease. As people go from left to right, they are progressively losing their insulin-producing capacity. They are becoming more and more insulin-dependent. Their immune system in their body is very active. Now we are able to identify this and there is an obvious genetic risk component to it. There is a robust effort to understand the genetic risk markers of all populations and all ethnicities along with all genetic makeups and their immunological markers. In addition to the auto-antibodies that we currently measure in the blood that are established markers of an active immune system, there could be other upstream markers that we need to identify early on, even before these autoantibodies are detected in blood. Also, T1D has a metabolomic manifestation. We need to track glucose levels and hemoglobin A1C levels in addition. There are now markers that can show damage to the insulin-producing cells. Breakthrough T1D has a whole effort that involves working with several partners – academic and industrial – along with other nonprofits and Federal funders. This effort is trying to collectively understand the best diagnostic and prognostic tools that we can hone in on to enhance our current practice, which relies entirely on our antibody levels, and what is called an oral glucose tolerance test. There are many statistical tools like continuous glucose monitoring metrics and other things that we should be leveraging in a broader scale, and then roll it out so that they are covered by payers and be available to people.
There are millions of people around the world already living with the disease. We have done significant work to offer them better ways of controlling their glucose levels, even delaying complications, but there are ways we can provide the insulin independence and that is through the cell therapy approach. All of the cell replacement therapies are from the seminal work in organ pancreas replacement, as well as islet replacement therapies. Islet cells are pancreatic islet cells, insulin-producing cells that are derived from deceased individuals or cadavers. Cadaveric islet transplantation, which is practiced in many parts of the world and recently was approved by the FDA, has demonstrated that replacing the lost cells through external sources, we are able to provide insulin independence, almost normal glucose functional levels, and potentially avoid long term effects of complications and reverse the disease. In some ways this is a practical cure, or a functional cure as it is sometimes called. Breakthrough T1D is working feverishly through research and development at the discovery level, testing then translating that into therapies and clinical trials, working with the industry to develop these therapies and then working with regulators, the FDA, and others to have a pathway. These therapies are reviewed in an accelerated fashion by regulators. There is an accelerated pathway for these therapies. Once approved, we seek insurance coverage and clinician education, so they can deploy these therapies to the individuals that need them, as well as make sure that people understand the benefits and risk profiles and avail the therapies. This is going to be a marathon.
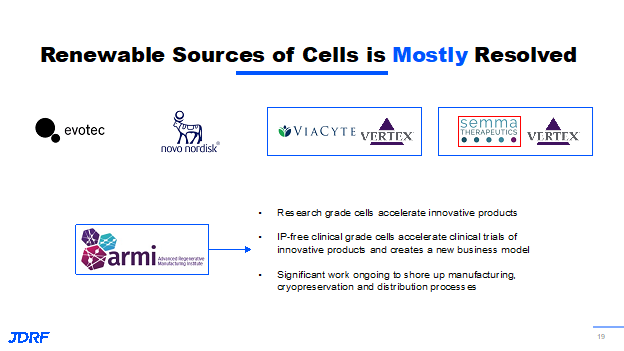
Cadaveric islet transplantation is already proven to work. There are still two essential problems, and this is what we are trying to address. One is a supply and demand issue, as we will never have enough cadaveric islets for the millions affected by the disease already. We need to produce these cells and collaborate the sources with others. Breakthrough T1D has always been part of stem cell biology. There have been 15 years of Breakthrough T1D funding along with other funders, including pharmaceutical effort, in developing these insulin-producing beta cells from non-beta cell sources outside the box in a future version of flasks in a test tube, then scaling them up to making them in quantities of millions. We can make sure that they are available for the masses affected by the disease. We know a problem with transplantation of any organ or any type of cells, not just pancreatic beta cells or islet cells, is that these cells are again vulnerable to the body’s immune system and are going to be recognized as foreign cells and be destroyed as they are in people with autoimmune diabetes. Breakthrough T1D has a robust effort in trying to protect the transplanted beta cells. The goal is to have them survive longer so they continue to make insulin and continue to illustrate the benefits that we are hoping to achieve.
The first problem is making cells, growing them in large amounts, and making sure their function is mostly resolved. Here are some examples of Breakthrough T1D funding basic research, clinical research, and working with the pharmaceutical companies both in the foundation and the T1D fund. These cells are actually in people and showing benefits. Breakthrough T1D knows the path does not stop there. We need to constantly foster innovation. We need to make sure that several other companies and large companies that are coming up with protection strategies are able to have the cells because we do not want everyone reinventing the wheel trying to make their own stem cells and then trying to grow them in large amounts. It is important to have a partnership with the advanced Regenerative Medicine Institute in New Hampshire, which is a regenerative medicine not for profit. It is significantly funded by the Department of Defense in multiple areas of regenerative medicine. Breakthrough T1D is now partnering with them to scale up the manufacturing as well as the clinical research that will eventually turn into insulin-producing beta cells so we can get more and more innovation, and more and more therapies that can be developed at a very fast pace.
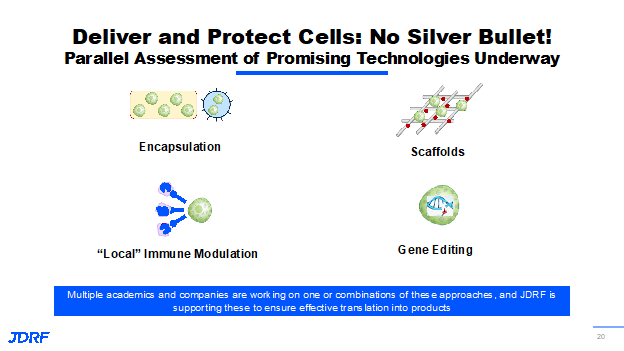
There is very important work that is being done by Vertex with the size of its beta cell devices along with companies like Novartis that are pursing innovations for T1D. The important item is to figure out how to protect these transplanted cells. Breakthrough T1D has helped to fund significant progress with many methodologies and technologies that we are working on and with others. There are approaches where we create scaffolds that are not fully encapsulated, but would require entire body immunosuppression. Research is ongoing to provide local immunosuppression at the site of implantation, so that the other organs are not exposed to the suppressive agents. An exciting possibility is the use of gene editing technology, which won the Nobel Prize a few years ago. Gene editing technology has been pursued by multiple disease research organizations that have organ failure as an endpoint. We hope to repopulate these beta cells through gene modification so they continue to make insulin, but are invisible to the body’s immune system. The idea is that they can be injected into the body, but immunosuppression does not need to be provided because these cells will behave like the body’s own beta cells but will not be recognized by the body’s immune system. This is very promising; Breakthrough T1D has multiple irons in the fire and they all have various advantages. Pharmaceutical companies are also figuring out the best way of protecting the cells. With all of these efforts, we will be able to come up with technologies that can protect the cells. It may be a combination of one or more of these approaches and at the end of the day, there will be several different products for people with T1D. These things are possible due to many important things that Breakthrough T1D started funding more than a decade ago, along with the creation of the Beta Cell Consortium.
This slide shows various circles with a multidisciplinary team that Breakthrough T1D has brought together. Breakthrough T1D has many on the team that have academic expertise, cell biology, genetics, immunologist and biomedical engineers. Breakthrough T1D also brings those with regulatory and policy expertise along with pharmaceutical companies and not-for-profit organizations. Many of these think tanks come together at least twice a year to meet in person, but also meet regularly throughout the course of the year to discuss the advances, discuss the areas of collaboration, avoid redundancies, and therefore be economical. Many things have been borne out of this consortium. They are listed on this slide with a few in the top right panel.
Examples are gene editing approaches, 3D bioprinting approaches to create these scaffolds and structures that can mimic the pancreas. Breakthrough T1D is working on how we can have novel immune engineering and immune imaging approaches. Many industries have benefitted from this consortium, and some of those are named at the bottom right. This is not an exhaustive list.
Another breakthrough is the recent approval by the FDA of cadaveric islet cell transplantation in the month of June. This is a particular process that happens at many research centers in the United States and in many clinics around the world. What the FDA approved recently was one such collaborative pilot transplant product. Breakthrough T1D is looking at the bigger picture, while this milestone often occurs with islet transplantation funded research by the National Institute of Health (NIH), Breakthrough T1D looks at this as a stepping stone toward many other therapies with stem cell replacement therapies. This paves the way to create a regulatory path forward for the type of clinical trials that need to be done for approval. This has been a long-term effort. The Breakthrough T1D research and advocacy department have collaborated with external experts in the field and funders like the NIH to get to this point. Breakthrough T1D is also heartened by the significant investment by the pharmaceutical and biotech sectors. This slide shows three examples of this.
To the left is something that you have heard about from the company Vertex which is now acquiring Viacyte, the company the Breakthrough T1D funded for many years. They have shown tremendous progress, including their stem cell-based therapy that is providing benefits in the VX-880 product, which requires immunosuppression. This is meant for the subset of the people with T1D that have an extreme condition called hypoglycemia unawareness. The results have been very promising so far and have been showing insulin independence in a few individuals that have had the therapy for more than a year, as well as showing the safety of this particular therapy. Vertex has not stopped there. They have another trial that just started, in which they are using their proprietary encapsulation technology without the need of immunosuppression and putting the same stem cells that are used in the VX-880 in a product that is called the VX-264.
There are still unmet needs. People with T1D are certainly doing much better than they were 10 years ago and certainly 20 years ago. If you look at their glucose control levels, we look at the A1C levels. If you look at the rate of reduction of complications that is happening, that is already encouraging. The awareness is there and the effort to do better is also up compared to what it was 10 ears ago. But the reality is that there are still unmet medical needs. Despite these advances, the average agency in the country is nowhere near where the American Diabetes Association (ADA) recommends. Two out of five adults are achieving those and then fewer in the pediatric population. There is also a huge mental health burden. We need to do more. Our strategy is trying to fill those knowledge gaps, by filling those product development gaps. This compliments the efforts going on by the rest of the team at Breakthrough T1D. The focus continues to emphasize a device-based approach. These devices have done well for people with diabetes in terms of glucose control, insulin delivery, automation, simplification. and miniaturization. Breakthrough T1D has been also steering in other therapeutic areas that can complement the action of insulin, provide help with better glucose control, and provide better metabolic control. Now we are seeing many of these adjunctive therapies that can provide a long-term benefit in delaying complications and providing benefits for heart and kidneys. They are actually approved for many people with type two diabetes and kidney disease, or people that do not yet have type two diabetes, kidney, or heart disease. They are not approved for those with T1D, though. T1D is the disease left out in these trials, by most pharmaceutical development. We are trying to change that and the role Breakthrough T1D can play in leading this effort. Breakthrough T1D is not forgetting the fact that this is a 24/7/365 disease that affects the individual, the immediate caregivers around that individual. and society at large and their mental health burden. The poor mental health and cognitive outcomes, as well as the clinical outcomes, are something that absolutely need to be addressed. The advances in closed loop systems, artificial pancreas systems, and better devices are all very encouraging. However, the large majority of the population are not achieving the target levels. Also, it is known as more of the population has become overweight or obese, people with T1D have an even harder time with their metabolism or glucose target levels. We know that two thirds of adults are overweight or obese and close to half of the people below the age of 18 in the pediatric and adolescent population are also overweight or obese. That has consequences beyond just T1D. Heart disease remains the primary cause of death in adults with T1D. There is a life expectancy gap between people with T1D and those without. The role of Breakthrough T1D is to close that gap in life expectancy and we will not stop until we get there. The Breakthrough T1D advocacy team is also making sure the Breakthrough T1D funding breakthroughs are covered and that there are options for everyone. As already mentioned, there are adjunctive therapies that are working wonders for people without T1D. Breakthrough T1D has funded studies that also show people with T1D have benefitted from these adjunctive therapies. There are two classes of these therapies. One is a class called GLP1 receptor agonist, like Ozempic , a drug that has been on the news a great deal. The other one is an oral therapy that helps with kidney and heart complications, and certainly with glucose control. This is called a sglt inhibitor that helps eliminate glucose through the urine output. These classes of drugs show significant progress. Breakthrough T1D is making sure that these therapies are available for people with T1D. That means getting them approved by the regulators that are covered by the payers, and people are actually benefitting.
There are multiple ongoing efforts with screening and measuring risk, like checking for autoantibodies and glucose levels. Here there are measures in assessing the psychological stress or mental health stress and support this through testing and participation in clinical trials. Breakthrough T1D has a robust program in training professionals that are specialized in providing the psychological care for people with diabetes. Work is being done to increase this area of specialization and to determine how can we have a healthcare model so we can implement this. It is not enough to develop these and get the data information from clinical trials. We need to have them covered and have a healthcare system that makes it easy for the healthcare provider and the person with diabetes. In this field, Breakthrough T1D appreciates additional funders like the Helmsley Charitable Trust, along with many others that also have robust impact in this space.
Clinical trials are the only way we can develop these therapies, get them approved and covered. Clinical trials take a very long time and much recruitment of people; retention of these people in the trials is long. There are multiple efforts ongoing to break down the barriers and hurdles preventing participation in clinical trials. Breakthrough T1D has a very successful clinical trial participation tool. There is also a robust program in educating people and empowering volunteers, along with empowering professionals to know about these therapies that are in development to know their risk level to know that they can actually benefit by participating in a clinical trial. Clinical trials are designed to be safe and effective. Breakthrough T1D has a patient advisory council which is a body of professionals with various backgrounds such as clinicians and nurse practitioners that are there to answer questions. Collectively, there are the clinical trial connection tool and clinical trial education volunteers, educational healthcare providers that Breakthrough T1D is deploying to help address education about clinical trials. Breakthrough T1D does need you as the champions in clinical trial participations so the answers to curing and providing better treatments happens more quickly.
This last slide shows the promise of the work Breakthrough T1D has been involved in funding. In blue above the line and the horizontal line are the therapies that are in the improving lives area, which are being tested. The black in the line below the horizontal line in the middle are the therapies that are here. If you go from right to left, these are the most advanced where we are having clinical trial results coming out currently. These therapies are in the cure space of disease modifying therapies, as well as cell therapies. In adjunctive therapies, the area of improving lives, these results are also imminent in the next few months. There are therapies that are just starting their clinical trials by the end of Breakthrough T1D’s fiscal year. This is basically a time horizon where near term is to the right, and a little bit of a longer term is to the left.
There is a lot to look forward to in the next few months and certainly through the next couple of years.
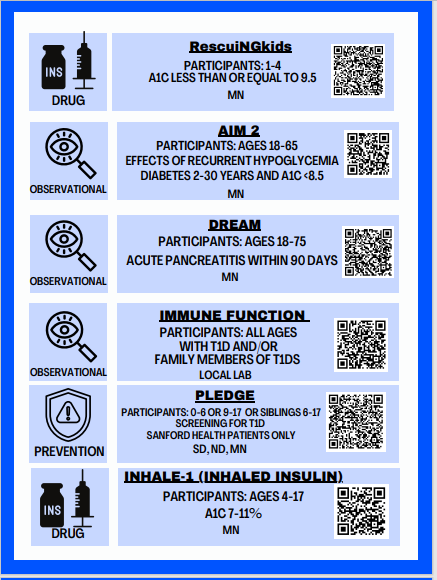
Clinical Trial Matching Tool and Clinical Trials available in our chapter: