Disease Modifying Therapies 2023
This is a recap of the research information talk (RIV) that took place earlier this year and was presented by Cassandra Bazile, PhD and Jay Tinklepaugh, PhD. Both are Breakthrough T1D scientists.
JDRF’s cures program research consists of three areas which are: global universal screening, disease modifying therapies (DMT), and cell therapies. These therapies are aimed at every stage of T1D (type 1 diabetes), from stage 1 where an individual has normal blood sugar and starts to express autoantibodies all the way to stage 4 where an individual has established T1D. Autoantibodies developed before or during stage 1 diseases are used to screen for T1D and screening is extremely important for the diagnosis of T1D. The overall goal of the DMT program area is to prevent, halt, or reverse the loss of beta cells. This can be done by using therapies directed toward the immune system, beta cells or both.
The focus of the DMT research strategy is twofold. The first strategy is to turn off the autoimmune attack against beta cells; the second strategy is to create and sustain beta cells. The ultimate goal of this is to develop therapies that both control autoimmunity and preserve or grow beta cells. Breakthrough T1D is playing an active role in accelerating the development of these therapies and reaching clinical testing, then eventually getting to the stage of regulatory approval and access. In the immune portfolio, the focus is on determining how to turn off the autoimmune attack against insulin-producing beta cells. The primary function of the immune system is to determine what is part of the body and what is not part of the body. The breakdown in the ability of the immune system to determine body from non-body can lead to autoimmune disease, such as T1D. It is important when creating therapies that we maintain a balance so that the immune system provides enough surveillance and recognition to identify and eliminate threats, but refrains from being so active that it starts to recognize and attack its own body because it perceives it as a threat. In the stages of T1D, autoimmunity happens long before stage 1, and it is confirmed by the presence of auto antibodies to self-tissue. This means that the turning of the immune system to your own cells happens prior to stage 1. It is important to not only understand what causes activations of the immune system, but also to develop therapies that can stop immune activations, as well as therapies that treat every stage of disease.
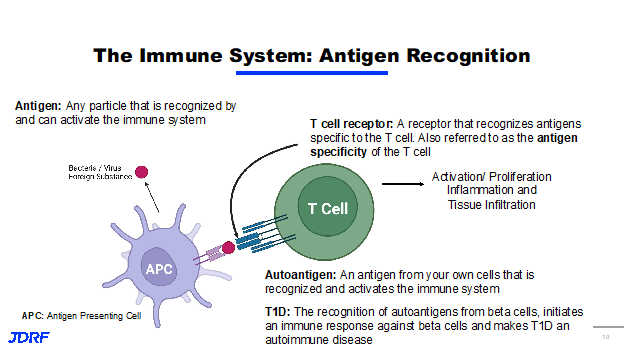
In talking about immune activation, it means antigen recognition. An antigen is any particle that can activate the immune system. We generally think of this as being bacteria or a virus that needs to be eliminated, but it can also be toxins or proteins. The immune system has cells that can present these antigens, called antigen presenting cells. They identify these antigens and present them to the T Cells. T Cells are a lymphocyte, which is a type of a leukocyte (white blood cell), that is part of the immune system. T cells recognize antigens through the binding of the antigen receptor called the T cell receptor. The antigen a T cell can recognize is determined by the specificity of the T cell receptor. This is called antigen specificity. Think of a key and a lock, for example. Only one key is specific to a certain lock. Once a T cell recognizes an antigen, the T cell then becomes activated, where the T cell can proliferate causing inflammation. When the antigen being recognized by the immune system comes from cells in your own body, this is called an autoantigen. In terms of T1D, the recognition of autoantigens from beta cells initiates an immune response, damaging the pancreas and leading to disease. Our understanding of the immune system has recently led to a huge breakthrough for T1D after decades of research and funding from Breakthrough T1D.
In November of last year, TZield, known as teplizumab, was the first approved DMT to treat T1D. TZield works by targeting and altering T cells, is approved for people with stage 2 T1D, and delays progression of onset for an average of three years. There is currently another study underway called the PROTECT trial that will test TZield and its ability to slow down the loss of beta cells and preserve beta cell function in children and adolescents with stage 3 or clinical T1D. There have been many clinical advances that are targeting the immune system to treat T1D.
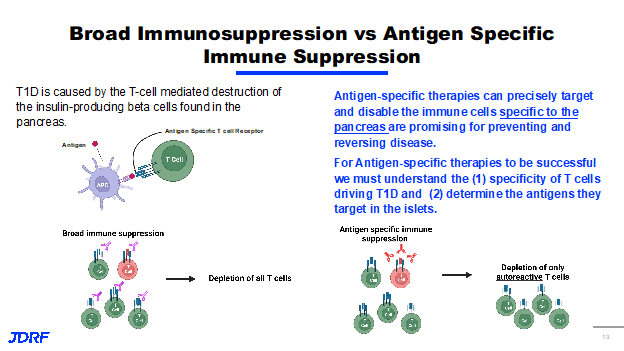
The immune system strategies for DMT for T1D consists of three primary areas: to stop the autoimmune attack, to enhance regulation, and to disrupt inflammation. Clinical progress has been made in each of these areas for stopping the autoimmune attack. The approval of teplizumab, as well as its testing of several other agents, has shown significant progress for enhanced regulation or approaches such as bolstering Treg function. Tregs are the T lymphocytes that regulate or dampen the inflammatory response. This research has been clinically proven to be safe; however, there still needs to be work done on efficacy. Finally, efforts that disrupt inflammation, such as using agents geared at cytokines, have showed promising clinical results. Cytokines are small proteins that take part in cell signaling in the inflammatory process. Recently, there have been several advances in technology that have provided Breakthrough T1D the ability to develop therapies that can further enhance the efficacy of drugs aimed at stopping the autoimmune attack and distrusting inflammation. One way Breakthrough T1D is doing this is by funding research in the development of targeted immune therapies. With recent success in therapies targeting components of the immune system, we may need to get more specific with the types of cells we target for safety and efficacy, as it pertains to both sides of the DMT portfolio. We can use targeted therapies for immune cells to identify antigens and receptors that cause disease. We can target just immune cells that drive disease to avoid a broad immune suppression. We can also use targeted therapy to sustain beta cells so we can deliver regenerative or proliferative therapies, especially to beta cells, avoiding off-target effects. Together, the goal of these approaches is to develop safer and more effective therapies that can be used to stop the autoimmune attack, promote beta cell health and survival, or ideally both in combination. T1D is caused by T cell mediated destruction of the insulin-producing beta cells in the pancreas. There are other cells that have been shown to have a role in T1D. Therapy that disabled T cells have been shown to be extremely effective in preventing and treating T1D. We know that T cells express the T cell receptor and these T cell receptors recognize molecules called antigens, and depending on the antigen specificity of the T cell, they can cause autoimmune disease. Current immune therapies utilize broad immunosuppression, which means that therapy targets a broad population of cells, no matter the specificity. Broad immunosuppression is represented in this diagram.
This shows T cells with different antigen specificity, with the red T cells recognizing self-antigens causing them to be autoreactive. In this example, broad immunosuppression leads to the deletion of all T cells, no matter their specificity, including T cells that are not involved in autoimmune disease. In contrast, additive specific therapy only targets the cells that have been shown to cause disease. Here it shows the same figure with the red T cells being autoreactive. In the cases of an antigen-specific therapies, the therapy will only start the autoreactive T cells, leading to depletion of only the autoreactive cells and thus allowing the non-autoreactive cells to survive. T1D specific therapies can precisely target and disable immune cells and are promising for preventing and reversing disease. However, for antigen-specific therapies to work, we must first understand the specificity of T cell driving T1D disease. Second, we must be able to determine the antigens that T cells are targeting. Breakthrough T1D is currently funding several investigators who are using unique technologies to identify autoreactive T cells as well as their antigens so that they can be used to create therapies. Dr. Maki Nakayama, PhD at the University of Colorado is pursuing the development of a technology that determines the antigen specificity of T cells from people with T1D across all stages. Her lab has established a screening system that takes T cells from blood islets in lymph nodes of donors and combines them with newly identified antigens from the pancreas. Upon activation of these T cells with islet antigens, the T cells emit a light, allowing for her lab to identify T cells responding specifically to islet antigens. She will use this platform to test antigens, allowing us to gain significant insight to the types of T cells that initiate disease, as well as provide potential ways to target these T cells using new therapies. Another lab that is working to identify the specificity of T cells in T1D is the lab of Dr. Michael Betts, PhD at the University of Pennsylvania. The Betts Lab has developed a strategy to study the direct interaction between T cells and islets from the same donor. This work is especially unique, since for the first time he will reproduce a system that happens in humans in an in vitro system.
The Betts Lab will isolate T cells and islets from the same donor and through a co-culturing system, we will be able to identify which T cells respond to antigens from islets. This work will allow us to understand how autoreactive T cells interact with islet cells to cause their destruction and potentially reveal novel pathways and mechanisms that can be targeted for drug development. Lastly, Dr. Stuart Mannering, PhD, from the St. Vincent Institute in Australia, is focusing on discovering new antigens. The Mannering Lab was previously part of a collaborative group that identified a new class of antigens that T cells respond to. The antigens that his group discovered come from stress beta cells, and they are able to activate T cells and from people that have T1D. This makes these T cells likely to play a role in disease. The Mannering Lab is now working to identify new antigens, utilizing a system that uses yeast to express islet antigens. The identification of antigens is essential for the development of assays to not only screen for autoimmune disease, but to create antigen-specific therapies to prevent or reverse T1D. This is an extremely exciting time for the disease modifying therapies project area. Breakthrough T1D is funding a range of approaches that utilize unique technologies to identify and discover new antigen specificities. The development of antigen-specific therapies offers the opportunity to target pathogenic cells driving T1D instead of broadly suppressing the immune system. While therapies that target the broad immune system can be effective for preventing and treating T1D, it seems that the therapies may serve as another opportunity for a safer and more effective treatment. Breakthrough T1D currently has multiple shots on goal to lead the development of antigen-specific T cell therapies.
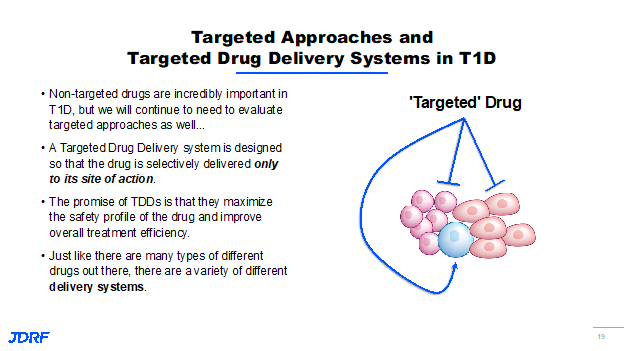
The next section is information shared by Dr. Jay Tinklepaugh. He works on DMTs with a specific focus on the beta cell. Breakthrough T1D is supporting projects that are centered on finding new ways to improve beta cell survival and function, identifying proliferative targets to encourage beta cells to proliferate, and implementing strategies focused on converting other cell types into beta-like cells. Each of these approaches has the potential to help restore insulin production in T1D and contributes to a lasting cure. Several of these beta cell-specific interventions will require targeted approaches so that these promising drugs are delivered only to the beta cell. Not all interventions will meet this targeting. Breakthrough T1D has several strategies that are being pursued to have multiple shots on goal in this area. Currently, we are seeing success with teplizumab, the exiting results from the Bandit trials regarding Jak inhibitors and verapamil. These non-targeted drugs are incredibly important. We continue to need to evaluate targeted approaches, as well. These strategies are targeted drug delivery systems that are designed so that any drug or intervention is delivered only to its site of action.
For example, as you see here, we want to ensure that the drug is only going to the beta cell (the blue cell in this example) and not to any of the others throughout the body. The promise of this approach is that it makes everything safer and decreases the amount of drug needed by being more efficient. This approach has been used successfully in the oncology setting for several years and has improved the efficacy of chemotherapeutic and radiologic treatments while reducing the terrible side effects of those treatments. We want to leverage the advancements made in that field for T1D. In order to accomplish this goal, there are a number of different drug delivery systems being looked at by Breakthrough T1D, including using beta cell-specific proteins as a form of drug shuttle with nanoparticles loaded up with regenerative therapies targeted to the islets to selectively reprogram cells. Both targeted and non-targeted approaches fit within the overall Breakthrough T1D strategy to impact the beta cell and T1D. One of the first lines of intervention that we can employ in T1D is simply to improve the survival and function of beta cells in response to stress and the attacking immune system. It has been found that not every beta cell dies as a result of the immune system attack in T1D. When beta cells are stressed, they can begin to lose function and ultimately die without direct intervention from the immune system. This is a compounding problem in T1D where we know the immune system is also causing widespread damage to beta cells as the disease progresses. One approach is to systemically deliver an anti-stress agent that provides a benefit to the beta cell. These anti-stress agents probably do not need to be targeted because of their relatively low safety profile. Previous Breakthrough T1D research has identified several different pathways and drugs that can keep beta cells alive and functional by making them more resilient to the immune attack. An example of a drug that does this is Verapamil. Verapamil is a widely used drug, primarily used to treat high blood pressure. However, in mice with diabetes, it was shown to reduce beta cell death which can see on this slide.
Mice who were not given Verapamil had more beta cell death and significantly less insulin production than those that received the drug, as you can see in the highlighted image on the right. Mice without Verapamil had no protection, whereas those who received Verapamil were protected. Breakthrough T1D has continued to support research on Verapamil, which has been assessed in several small clinical trials over the past couple of years. Currently we are awaiting the results of the first large-scale trial incorporating Verapamil and early onset T1D populations, with those results coming out later this year. The second approach is focused on finding ways to get the beta cells that have survived the immune attack and the stressed cells to proliferate, or multiply. The goal of this would be to replace the beta cells that were lost by encouraging the survivors to multiply and restore their overall number as seen on this slide.
For many years, beta cells were considered part of a small subset of cells within the body that are considered nonproliferative. Thanks to Breakthrough T1D funded research, there have been several exciting discoveries in this area over the years and we now know how to make beta cells proliferate in vivo. Unfortunately, there are still concerns that these proliferative strategies are not beta cell-specific and that they might impact other parts of the body. This is a problem because proliferating cells in the wrong parts of the body could create some major safety concerns. Proliferative approaches will probably need to be more targeted unless we can continue to work on identifying a drug that impacts the beta cell exclusively.
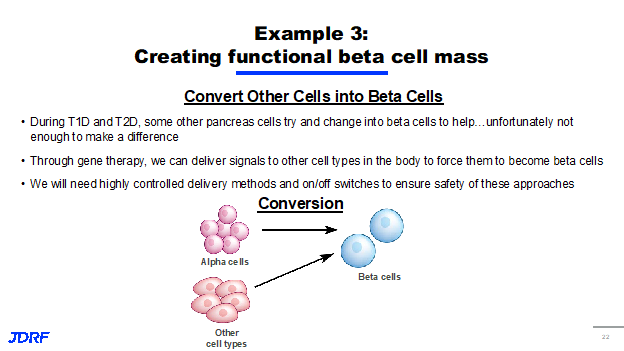
The third approach we are taking involves the conversion of one type of cell into something that much more closely resembles a beta cell. Research has shown that as T1D and type 2 diabetes progress, other cells in the pancreas try to change their metabolic processes to help out and supply insulin to the body. Unfortunately, not enough cells are able to change to make up the difference in people with T1D who still need to take exogenous insulin. We are currently researching ways to use gene therapy or chemical interventions to deliver signals to other cells both inside the pancreas and outside of it to force them to become more like beta cells. This means that they can respond to changes in glucose within the body and produce insulin. The hope is that this approach will serve to alleviate the need for exogenous insulin in people who have longstanding T1D. We will need to have a highly controlled delivery method as well as on and off switches for these converted cells to ensure that they are implemented safely, and so any potential cell conversion therapy will almost certainly need a targeted component.
One of Breakthrough T1D’s scientific projects is from Dr. Assam El-Osta, PhD at Monash University in Australia and is focused on creating functional beta cell mass. Dr. El-Osta was given an innovative award to examine whether a subtype of human pancreatic cells, called ductal cells, can be used to drive beta cell regeneration. These types of innovative awards are given to projects that are very risky, but have the potential to drive significant advances in the field. As part of this award and part of his earlier research, Dr. El-Osta has shown that the enzyme EZH2 plays a significant role in beta cell development. This work is examining whether chemical inhibition of the EZH2 enzyme can drive the conversion of the so-called pancreatic ductal cells or other pancreatic progenitor cells into insulin secreting beta-like cells. So far, the results look promising. In just the one year his team has shown that this strategy was able to restore insulin in the pancreas from a T1D diabetic donor. The results of this work were published last year. There is still a long way to go with this work and strategy.
The second project to discuss comes from the company Neurodon and Dr. Russell Dahl. It is focused on preserving functional beta cell mass. This award is part of Breakthrough T1D’s industry development partnership program. Dr. Dahl and his team at Neurodon are designing new compounds designed to prevent beta cell stress. These compounds are specifically designed to help mitigate metabolic dysfunction within the beta cell by activating a specific enzyme called SERCA2 in the hope that this will help them to function properly and contribute to better overall survival. This project also highlights a key role Breakthrough T1D plays in the research development pipeline, as previous funding on beta cell stress to researchers Dr. Evans Molina and Dr. Zurich laid the foundation for these advancements. Breakthrough T1D is uniquely positioned to drive progress from academia to industry and build these types of partnerships that will be key to finding a cure.
Another project is the exciting work being done by Dr. Paolo Serafini, PhD, to develop new ways to increase functional beta cell mass . Dr. Serafini’s work is focused on using nucleic acid aptamers made of either DNA or RNA to deliver drugs specifically to beta cells. What makes these short sequences of DNA or RNA so special is that they can fold into a shape that will only fit a very specific target. The analogy, again, is similar to a lock needing a specific key. The folding pattern allows the aptamer to bind tightly to its target. The drug can then be delivered with very high specificity. During this last year, Dr. Serafini and his team have made exciting progress on this platform and have generated additional evidence that these aptamers can deliver a therapeutic specific to the beta cell, leading to a high impact publication. These aptamers also continue to be evaluated and examined for their ability to deliver even more therapeutics and are prime candidates to be integrated with additional targeted drug delivery strategies focused on gene modification and immunotherapies.
In summary, current research in targeted drug delivery approaches is predominately in the proof-of-concept stage. Breakthrough T1D is working across the field to keep this research moving forward rapidly. However, there are still some major barriers to progress. The technology being developed is cutting-edge. There have been tremendous advances made with similar indications such as in oncology, but a lot of these things are new to T1D research so they will need to be validated and examined before we can fully take advantage of them. There are still a limited number of targets that are specific to the beta cell. The future for DMTs is extremely promising. Breakthrough T1D will continue to identify discovery research that focuses on new and expanding areas of immune intervention and continue to develop new combination therapies into research and development. This will be for both immune dysfunction and beta cell function. The research will continue to expand the number of targeted drug delivery approaches we have for both the immune system and beta cells.
If you have any questions on clinical trials in our chapter area, please contact me. I am the clinical trials education volunteer (CTEV) for the Breakthrough T1D Minnesota and Dakotas chapter. I can also help with the Breakthrough T1D matching tool and I can help with finding clinical trials. I am Debbie Evans and can be reached at debbieaevans1@gmail.com
You can also watch the above talk on the Breakthrough T1D YouTube Channel HERE.