Minnesota & Dakotas Clinical Trial List
Breakthrough T1D Clinical Trials Connection Tool
Type 1 Diabetes (T1D) is an autoimmune disease that attacks the beta cells in the pancreas. Beta cells in a healthy pancreas will produce insulin that allows glucose from the blood stream to enter cells in our body to provide energy from the food we eat. Scientists now know that the beta cells of someone with T1D are different from the healthy cells from the beginning, and contribute to the disease progression. In T1D, the pancreas cannot produce insulin, glucose cannot enter cells, and insulin must be injected. Management is not easy as the blood glucose fluctuates. This fluctuation can lead to short- and long-term consequences. The Breakthrough T1D goal of developing disease modifying therapies (DMT) is to restore the ability of the pancreas to produce insulin.
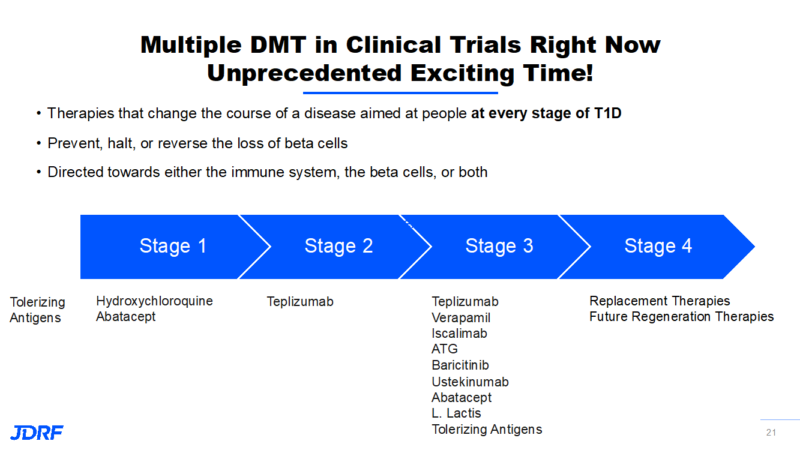
Our mission is to work toward universal screening to uncover those who currently have autoantibodies. Many who develop T1D did not know they had developed these autoantibodies. Screening is very much connected to halting and preventing the disease with the use of DMT. DMTs are meant to alter the course away from a disease trajectory. Currently, therapies that are aimed at people who are on the path to developing T1D are rare. We are now working on therapies that are aimed at stages 1 through 3 of T1D. Stages 1 and 2 occur when one or two autoantibodies are detectable, and stage 3 occurs when you are diagnosed with T1D and now must inject insulin either by shots or by an insulin pump. These new treatments are meant to prevent, halt, or reverse the loss of beta cells. Therapies target modifying the course of the disease, and are designed to correct the immune system or to help beta cells survive. These are treatments meant for all stages in a person who still has viable beta cells to regenerate. Research has found that it is still possible to have viable beta cells in those living with T1D, though it varies with each person and how long they have had T1D. The goal with these therapies is to turn off the autoimmune attack against the insulin-producing beta cells so the body can create new cells and sustain those new beta cells. Breakthrough T1D is accelerating the development of these therapies into clinical testing and then eventually, regulatory approval and access.
Today, Breakthrough T1D has about 10 active trials with new DMT. We see promise going into clinical testing and are working on getting these new therapies to patients. Breakthrough T1D has been funding DMT for years and the success of this funding is bringing us to the human clinical trials we are seeing today. We are working with companies to engage in this research. There are many new avenues of treatments being investigated.
Read more about the science of these new treatments:
First, we need to look at the disease mechanism. As the insulin-producing beta cells become stressed, they become more attractive to immune cell-mediated attack. The immune system is sophisticated with many cell types and substances secreted by the immune cells. When healthy, the various immune cells function together to keep a balance in our bodies and protect us from invaders. Here are the players of immune cells:
Effector T cells’ (Teffs) job is to recognize threats, such as cancer and infections. In T1D they erroneously see beta cells as a threat and attack them. In T1D they become too active, so Breakthrough T1D is working to lower their numbers and/or disable their activity.
Regulatory T cells (Tregs) are the counterbalance to the T effector cells. Their job is to cool down the activity of the T effector cells so they do not go out of control. This is to maintain a balance in immune regulation. In those with T1D, their numbers are too few and they are not robust cells. They are unable to protect beta cells.
Immune deviator cells secrete substances that encourage inflammation and make the beta cells attractive to the immune system. This leads to a hostile environment for beta cells and ultimately to beta cell death. As this happens, it makes the beta cells even more attractive to the immune system. These immune deviators support the Teffs too much and the Tregs not enough, so there is an imbalance.
Click on this handy chart for the above:
There are many new treatments that are entering the various phases of human clinical trials. ATG, or anti-thymocyte globulin, is being funded by Breakthrough T1D, the Helmsley Charitable Trust, along with TrialNet through the NIH. ATG inhibits certain types of immune cells. Breakthrough T1D’s work with ATG goes back 10 years, when it started studying this to see if it would dampen the immune attack and preserve beta cells. In the early 2010s ATG research by Helmsley Charitable Trust showed safety and the early mechanism of helping T1D in humans. In 2019 TrialNet, in a phase 2 trial, showed ATG promoted a two-year extension of the honeymoon phase and that it supported positive changes to the immune system. This helped delay the loss of beta cells in stage 3 T1D. Currently, Breakthrough T1D along with partners INNODIA, Sanofi, and SAB Biotherapeutics are continuing to work on ATG as a treatment. The key takeaway is that these combined studies, along with company engagement, show promise for ATG therapies to become a reality for the T1D community. EU funded INNODIA, a large T1D consortium in Europe, has decided to launch a trial for the lowest effective ATG dose. Sanofi produces the treatment ATG. The effect of Breakthrough T1D involvement will help bring these advances to patients in the coming years.
Teplizumab, an anti-CD3 antibody, will be the first-in-class therapy being developed for T1D that targets activated T effector cells and disables their activity when it is approved. Breakthrough T1D has played a critical role in bringing this therapy from discovery, 15+ years ago, to where we are today. Teplizumab is able to delay stage 3 T1D by three years when given to patients in stage 2 T1D. The FDA is currently reviewing this treatment application by the company ProventionBio for stage 2 T1D.
Ultra-low dose IL-2 (Interleukin-2) is promising for disabling immune activity that is harmful to beta cells. Low-dose IL-2 bolsters the function of the T regulatory cells (remember, they help calm the immune reaction) so they can help limit the harm done by the T effector cells (remember, they participate in the attack on the beta cells). In T1D, the application of this treatment is to increase the T regulatory cells to protect the beta cells, and to prevent damage done to the beta cells by the T effector cells. This has been a 15-year partnership with Wellcome Trust, Pandion Therapeutics, and the T1D fund. This year, Pandion has reported safety and mechanistic data for these next-generation IL-2 treatments that will only target T regulatory cells.
The drug ustekinumab (Stelara) is able to block multiple harmful pathways and has been proven safe in the treatment of diseases such as psoriasis and Crohn’s disease. There are several in this class of drugs that are being studied for use in T1D. In the efforts to find drugs that can be repurposed and effective in T1D, Breakthrough T1D has excelled in forming consortiums of scientists, funders, and companies that work together and share their progress to move things forward faster. Phase 2 trials on ustekinumab are still ongoing in the UK. There are current and future phase 2 and 3 trials in Canada. Breakthrough T1D will continue to assess and fund this research.
Presently there is a lot of excitement around the class of drugs called JAK inhibitors (JAKi). They are drugs you may have heard of that are used for some cancers and autoimmune diseases, such as rheumatoid arthritis (RA). Some examples of trade names/drug names are Xeljanz/tofacitinib, Olumiant/baricitinib, Jakafi/nuxolitinib, and Rinvoq/upadacitinib. T1D researchers look at this class of drugs to bolster the survival ability of immune system targets, such as beta cells, and also to inhibit the immune system. So far, we have been able to see that JAKis can keep beta cells alive in animal models that have T1D. Multiple clinically approved JAKis were tested to find the right ones that could help with T1D. Breakthrough T1D-funded researchers Dr. Helen Thomas and Dr. Thomas Kay have begun the first T1D trial with a JAKi in Australia using a drug produced by Eli Lilly. Dr. Helen Thomas discovered this class of drugs could prevent the beta cell killing. Breakthrough T1D feels that we can halt the disease progression if this is used early enough and prevent insulin dependence. Importantly while this research is ongoing, two case reports have emerged that help show why we think JAKis are important. The first is a 71-year-old woman with RA, systemic sclerosis, and T1D who did not respond to classic RA treatments. She was put on a JAKi to treat her RA, which improved her RA and skin sclerosis symptoms but also halted the progression of her T1D. This occurred in Japan. The second case report was of a 17-year-old boy with a history of untreatable inflammatory conditions who was diagnosed with T1D and presented in diabetic ketoacidosis. It was noted that genetically, he had a STAT1 mutation that could explain his multiple inflammatory conditions. Treatment with a JAKi decreased his inflammatory conditions, but it also returned him to insulin independence. This was reported in the New England Journal of Medicine (NEJM).
A small study showed that the drug Verapamil can block beta cell destruction induced by diabetes stress. This study found that Verapamil could halt T1D disease progression in recently diagnosed adults. Verapamil is an FDA approved drug for blood pressure, and has also been found to reduce cellular stress. In T1D research, Verapamil is found to work on reducing fatal levels of beta cell stress brought on by the immune attack and high blood sugar levels. Drs. Jennifer McVean and Antoinette Moran at the University of Minnesota are doing a clinical trial with Verapamil and a hybrid closed loop (HCL) system in very newly diagnosed T1Ds. This is the CLVer study. They are looking to see whether separately or in combination, this approach can halt or slow T1D progression in children and adolescents. Breakthrough T1D is also funding Verapamil with INNODIA, a European public-private partnership, to test different ages and stages of T1D. INNODIA partnered with Breakthrough T1D’s plan to pair Verapamil with other immune drugs to see if a combination works better than the treatments alone. These are trials that are using treatments that are already approved. Positive studies can help to put these treatments more quickly into the hands of patients.
In summary, there are many therapies Breakthrough T1D is currently working within all three stages of T1D. Click on the image below for more detail.
As we are able to see treatments work in various stages, researchers are working to move successful treatments into earlier stages of the disease. In order to be able to stop the progression of T1D before clinical symptoms occur, more people need to be screened. This is the time to be part of TrialNet if you have a relative with T1D, or to use the T1Detect kit that can let you know if you have developed various autoantibodies. The future of halting the disease relies on being able to know these autoantibodies have developed. Stage 3, which is better known as clinical T1D (requiring insulin injections), can occur at any age, but it tends to have the first autoantibodies before age 5. Of all new T1D diagnoses, only 10% had a family connection. The hope is that more people without a known connection will use the T1Detect kits and be able to prevent the disease if autoantibodies are detected.
Breakthrough T1D is working to prevent, halt, and reverse the loss of beta cells while also encouraging them to multiply. This goes along with protecting them from autoimmune attack. The hope is to also target a more localized immune protection treatment. The idea is to send treatments directly where they are needed. There continue to be many clinical trials in our chapter area with a variety of types of trials such as drug and device, along with others. Here is the chapter chart showing the active clinical trials. As always, if you have any questions please contact me, Debbie Evans. Cell: (612) 810-1933 or via email at: debbieaevans1@gmail.com.