Aiedan Mosley, a 16-year-old San Francisco native with autism spectrum disorder, enjoys football, track and field, music, and movies. He can play tunes by ear and loves Journey and old-school rap. Aiedan aspires to be a musician, actor, and film producer, like his role model Ice Cube. “Aiedan has worked so hard to be where he is today, and we are so proud of all the hard work,” said his caregiver, Jessica.
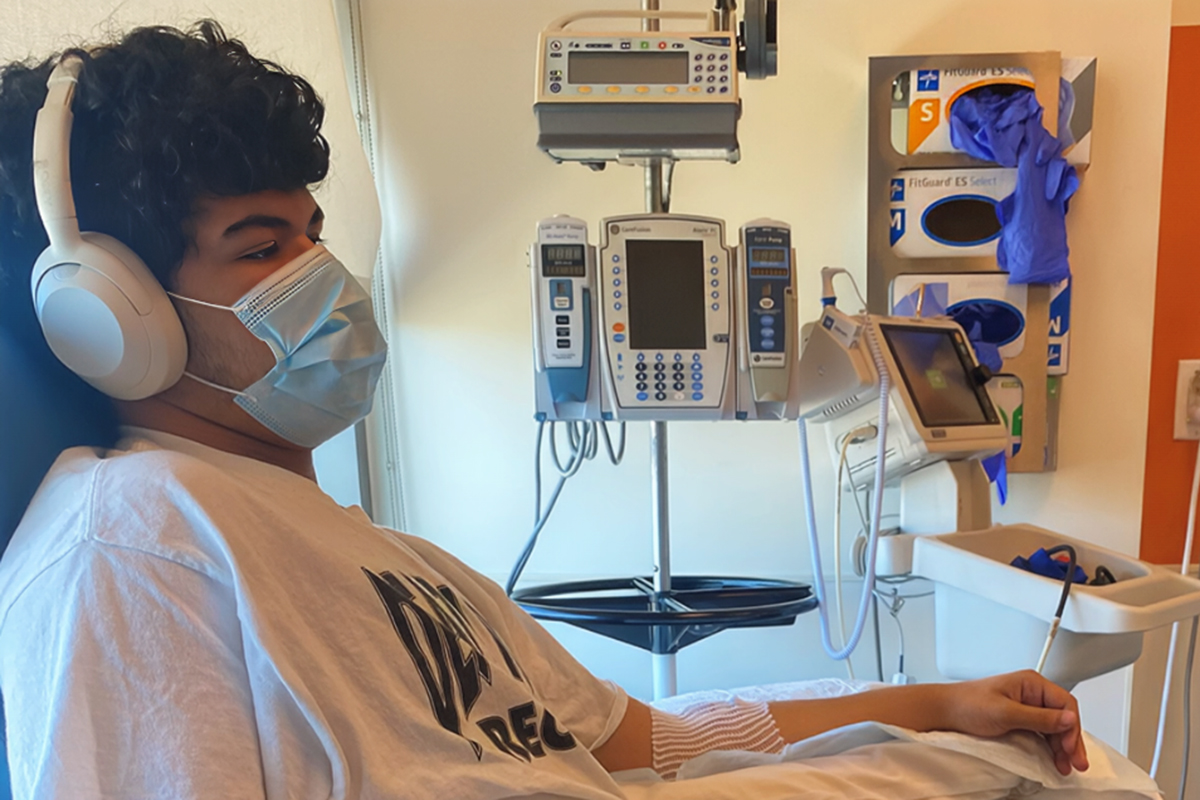
Typical high school life changed in June of 2024. Jessica noticed differences in Aiedan’s behavior, including mood swings, tiredness, and weight loss. Worried, she scheduled a visit at the University of California San Francisco (UCSF) Adolescent Health Clinic. The day before, Aiedan was so thirsty that he drank four glasses of water before dinner. After the appointment, a call from the doctor revealed that Aiedan’s blood glucose was 550 mg/dL—they immediately rushed to the emergency room.
“Aiedan was admitted for 3 days, and we got a quick tutorial into the world of type 1 diabetes (T1D). Nothing can prepare you or your child for the finger pokes and the insulin shots,” explained Jessica. The diagnosis came as a shock since they had no family history of T1D.
Soon after, Jessica started researching clinical trials, curious to know if there was a way to preserve Aiedan’s insulin production. Feeling unsure, she spoke with Breakthrough T1D for additional support and had an honest conversation with Aiedan. “He said he wanted to make a difference and find a cure,” said Jessica. Aiedan agreed to participate in TrialNet’s T1D RELAY Study.
TrialNet’s T1D RELAY Study: A new combination of two FDA-approved immune therapies
TrialNet, the largest T1D clinical trial network in the world, is aimed at preventing T1D and stopping disease progression by preserving insulin production before and after diagnosis. TrialNet is recruiting people newly diagnosed with T1D for a clinical trial (T1D RELAY) that will evaluate the combination of two FDA-approved immune therapies, rituximab-pvvr (Ruxience®) and abatacept (Orencia®), in maintaining insulin production. Both are FDA-approved for other autoimmune diseases such as rheumatoid arthritis.
Stemming from earlier TrialNet studies in which each therapy alone delayed insulin loss, this trial will assess whether abatacept given after rituximab-pvvr will better preserve insulin production compared to rituximab-pvvr alone. Because this strategy has shown promise in rheumatoid arthritis, researchers are confident that this may be a successful approach for T1D.
“Clinical trials are how we find cures”
Aiedan is handling the trial like a superstar. He experienced mild side effects from rituximab-pvvr, but his biggest challenge was keeping up with missed schoolwork because of lengthy infusion days. Now, Aiedan is receiving either abatacept or a placebo at home and has had few hyperglycemic events since the start of the trial.
For families interested in exploring clinical trials, Jessica’s advice is to do your research and be realistic with your child. “Aiedan listened to me and the [trial] coordinator, and he made the choice to move forward with the study knowing it could be hard at times; in the end, it will benefit not only him but many others,” said Jessica. “Clinical trials are how we find cures.”
Support the T1D community by participating in clinical trials
Clinical trials are key to pushing medical advancements forward so we can get therapies to people with T1D quickly and safely. By choosing to participate in a clinical trial, you can directly impact the T1D community by helping to accelerate treatments and cures.