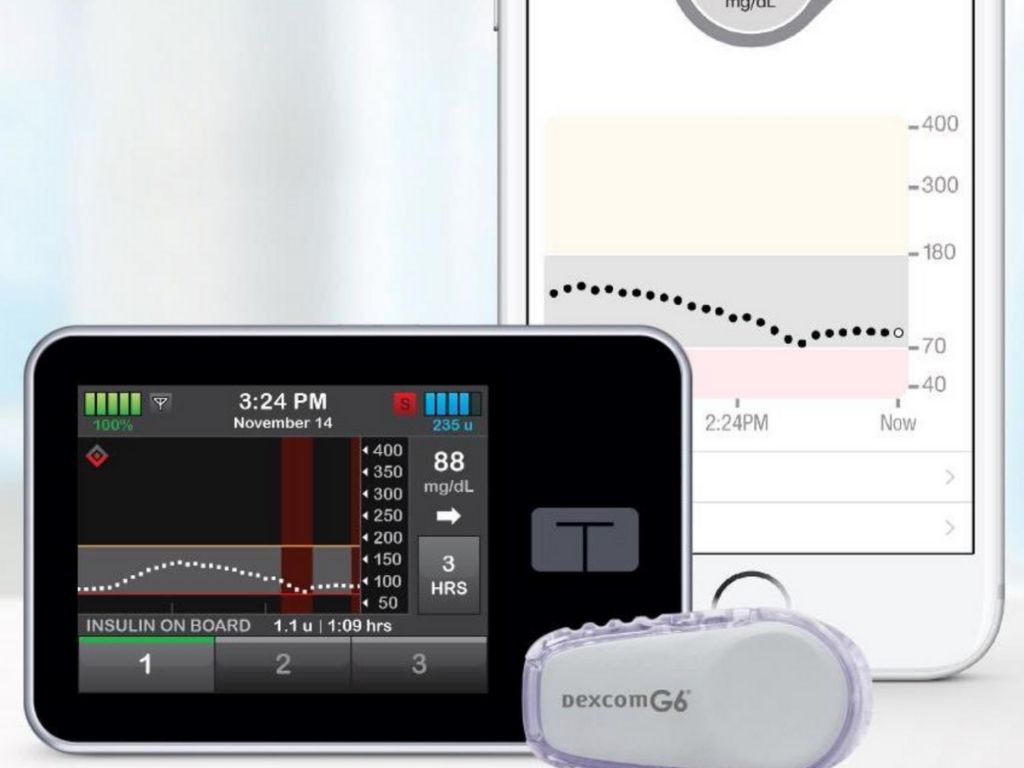
A clinical trial at four pediatric diabetes centers in the United States has found that a new artificial pancreas system—which automatically monitors and regulates blood-sugar levels—is safe and effective in children as young as age six with type 1 diabetes (T1D): Tandem’s t:slim X2™ insulin pump with Control-IQ™ technology. The algorithm in the Control-IQ technology came out of work by Breakthrough T1D-funded investigators, Boris Kovachev, Ph.D., and Marc Breton, Ph.D., at the University of Virginia.
This device was initially only authorized by the FDA for use in people 14 and older; the FDA amended its authorization to children 6 and up in June. Results from the trial were published in the New England Journal of Medicine.
“The publication of this landmark study provides proof that artificial pancreas systems work in children at a very young age,” said Sanjoy Dutta, Ph.D., Breakthrough T1D Vice President, Research. “Families now have an additional option to explore as they seek to find the system that best suits their needs, and this is a big win for the T1D community.”
The International Diabetes Closed Loop Protocol-5 (DCLP5) clinical trial recruited 101 children, ages 6-13, to take part, and the results are definitive: Control-IQ helps children with T1D do better. Per the publication, children experienced:
- Increased time in desirable glucose range (70-180 mg/dl) overall (67% for those on Control-IQ compared to 55% in the control group)
- Increased time in range overnight (80% for those on Control-IQ compared to 54% in the control group)
This study was funded by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) with funds from the Special Diabetes Program (SDP), for which Breakthrough T1D is the primary advocate.
Breakthrough T1D has been a leader in the development of artificial pancreas systems since starting the Artificial Pancreas Project 15 years ago. Through years of research funding, collaboration with regulatory agencies and leadership in the field, Breakthrough T1D has helped accelerate the development of transformative therapies that make life better for people living with T1D.
Breakthrough T1D’s goal from the beginning was to support multiple artificial pancreas systems. Now two are on the market, and there are many more in development.
Breakthrough T1D has also been a leading advocate for coverage, affordability and choice for diabetes technology and the insulin people need through our Coverage2Control (C2C) Campaign. In a victory for the C2C Campaign—driven by our powerful network of advocates—the nation’s largest insurer, United Healthcare, announced that they will expand their insulin pump coverage to include the Tandem pump. People with T1D now have more choice in how they manage their diabetes, and we applaud United Healthcare for this change.
Read more about how we are advocating for coverage, affordability and choice.