While genetics play a major role in the development of type 1 diabetes (T1D), mounting evidence supports the strong influence of the intestinal environment—called the microbiome—in this disease. Nevertheless, few “microbiome-based” strategies have been tested in clinical trials. Recently, Graciela Lorca, Ph.D., professor of microbiology at the University of Florida, found that one bacterial strain—a probiotic called Lactobacillus johnsonii N6.2—prevented the disease in animal models. Dr. Lorca and Michael Haller, M.D., professor and chief of pediatric endocrinology, went on to test this unique probiotic in healthy adults. Having found that it was safe and associated with promising biomarkers, they developed a current clinical trial to determine the potential for L. johnsonii N6.2 to help children and adolescents with recently diagnosed T1D.

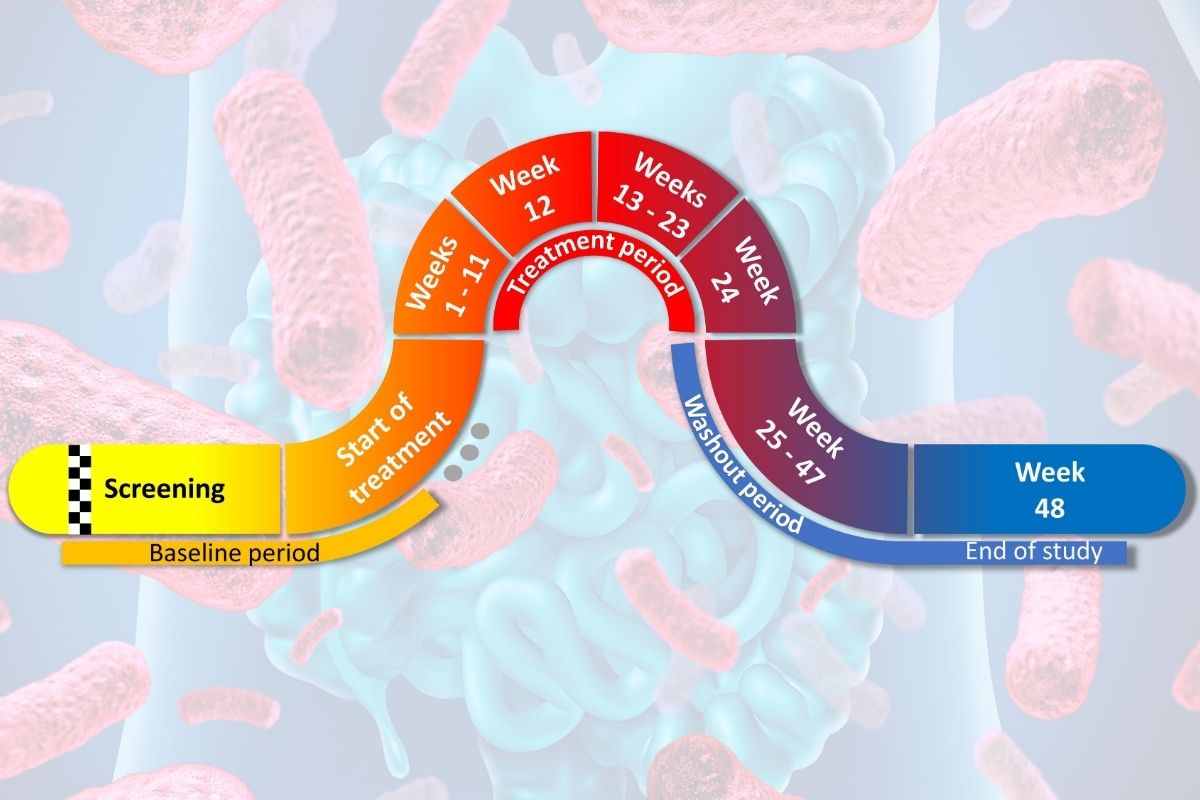
The trial, available only at the University of Florida, will enroll 57 children and adolescents, ages 8-17 years old, who have been diagnosed with T1D for less than 1 year. Participants will take a capsule containing L. johnsonii N6.2 (or placebo) for 24 weeks, and the researchers will explore the safety and immunological response to the drug. If the study is successful, the results will provide a strong foundation for using L. johnsonii N6.2 in prevention trials in subjects at-risk for T1D.
If you are interested in participating, please contact study coordinator Miriam Cintron, at 352-273-5580 or cintrm@peds.ufl.edu.