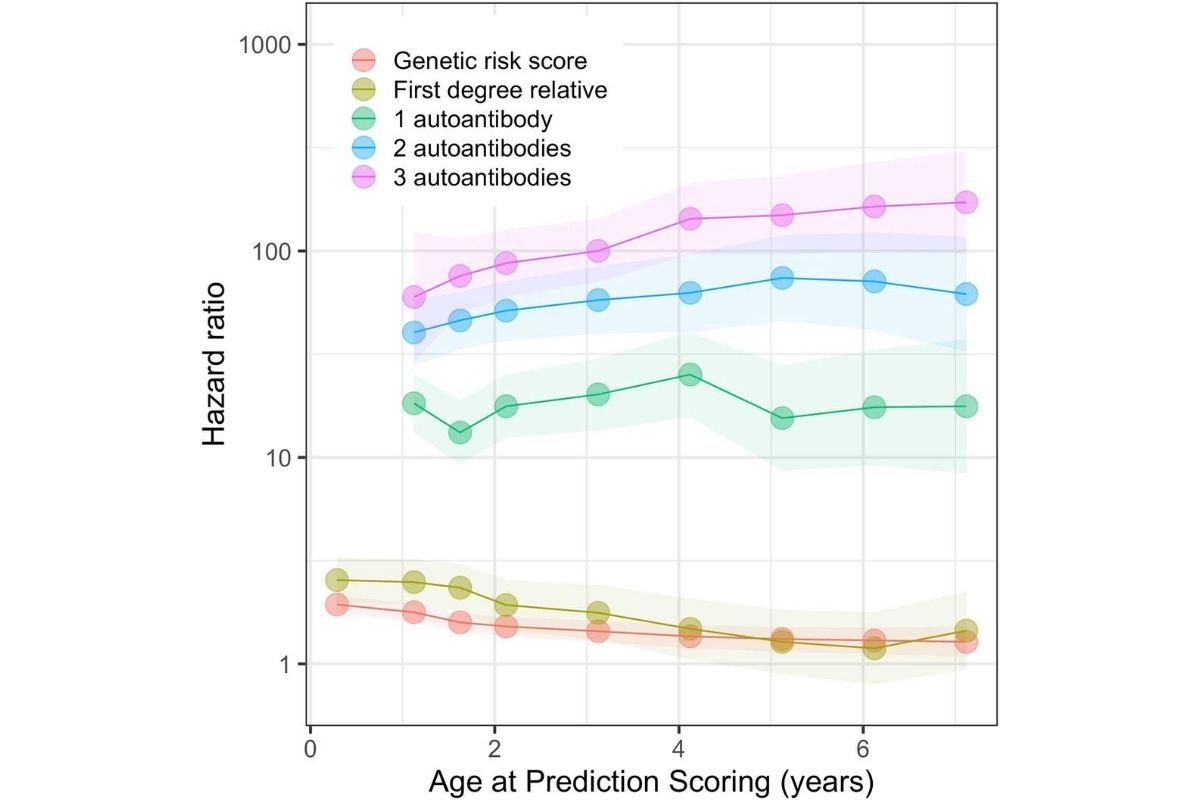
Type 1 diabetes (T1D) can be hard to predict in high risk children. Testing for the presence of T1D autoantibodies—antibodies against one’s own proteins—helps reduce the incidence of diabetic ketoacidosis (DKA)—a common occurrence in children upon diagnosis, that can lead to death if not treated—but this testing is not widely available. While having 2 or more T1D autoantibodies is pretty definitive that you will develop T1D, it doesn’t tell you when. Clinical diabetes can follow weeks after your autoantibodies appear, or even decades later.
But what if there was a way to more precisely estimate future T1D risk? Breakthrough T1D-funded scientists in The Environmental Determinants of Diabetes in the Young (TEDDY) study wanted to find out.
They used data from the TEDDY study—of nearly 8,000 high-risk children, followed closely from birth for 9.3 years—to develop a model predicting T1D during the first 10 years of life. They found that three variables were enough—autoantibodies, genetic risk score and family history. The combined risk score, put simply, identifies those children who require frequent surveillance because of the imminent onset of T1D.
This is tremendous for several reasons:
- This guides families regarding the risk of impending onset of T1D in their child, who can, among other things, prevent diabetic ketoacidosis—averting the (sometimes serious) brain damage, treatment costs and distress that it brings.
- This may allow for consideration of public-health based newborn screening for T1D, as well as related autoimmune diseases.
- It may greatly improve the cost and feasibility of early clinical trials, such as those testing expensive vaccines, by reducing the number of participants needed.
“Using T1D autoantibodies to assess risk and reduce the occurrences of DKA has long been a priority of Breakthrough T1D, and we are working to increase the availability of screening,” says Francis Martin, Ph.D., director of research at Breakthrough T1D. “The work of several members of the TEDDY team has demonstrated that, by including an aspect of genetic testing, you can further target your screening and education to those people who are at the highest risk and need—and is an important area for future research.”
Breakthrough T1D would like to see T1D screening become more readily available, be reimbursed by healthcare providers and become part of early childhood wellness programs. For more information, visit our Early Detection page.
In addition to Breakthrough T1D, the TEDDY study is funded by the National Institute of Health (NIH), as part of the Special Diabetes Program, which has been renewed by Congress numerous times thanks to advocacy and leadership by Breakthrough T1D. The Special Diabetes Program (SDP) is set to expire this year on November 30, and we need your help to urge Congress to renew this critical funding. Through the SDP, the National Institutes of Health (NIH) receives $150 million annually for T1D research. Please encourage supporters to become a Breakthrough T1D Advocate today.