Credit: Diabetes Care
In December 2017, the type 1 diabetes (T1D) community got a jolt, when a publication, testing the insulin concentrations in 18 vials, said that “none of the vials met the minimum labeled concentration standard.” In other words, insulin was not at the recommended dose; in fact, the percentage of insulin was around 40% (as opposed to a minimum required dose of 95%). Variation in insulin activity or insufficient insulin activity would pose significant challenges and safety risks for people with diabetes attempting to manage their blood glucose levels. So Breakthrough T1D, the American Diabetes Association (ADA) and The Leona M. and Harry B. Helmsley Charitable Trust issued a request for proposals (RFP) to verify the findings.
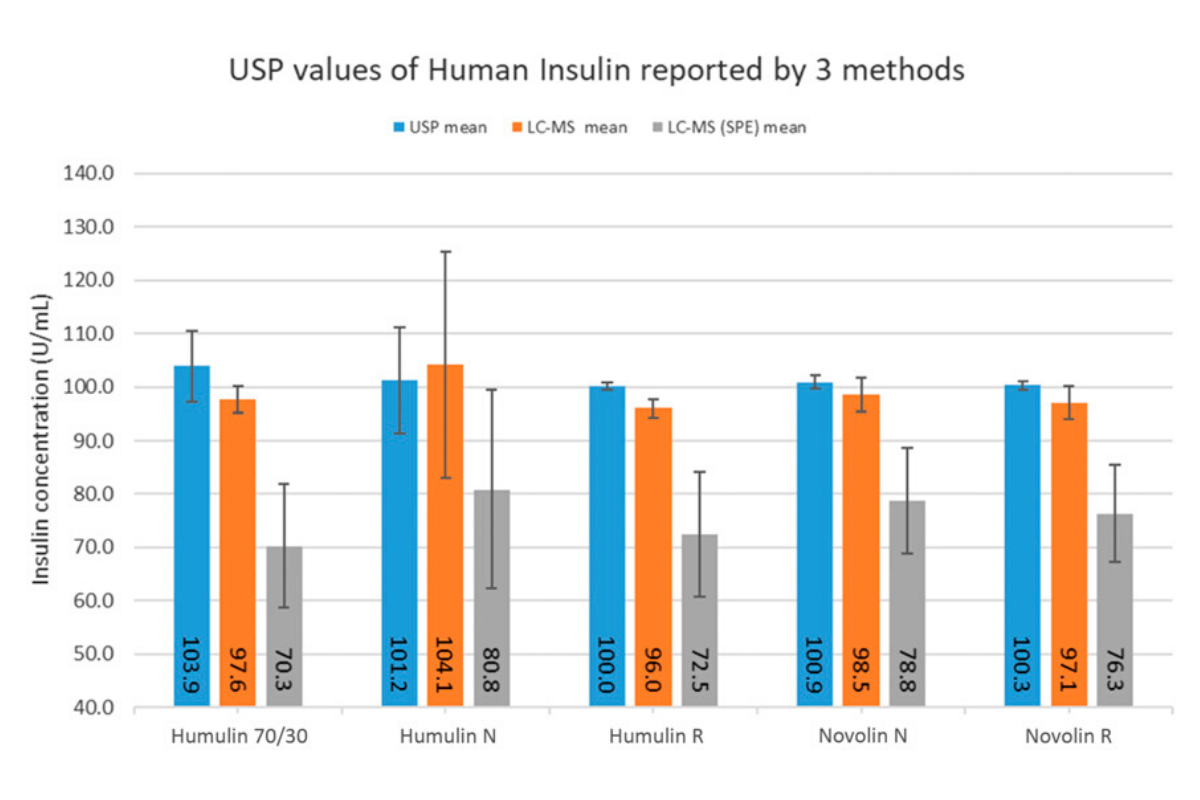
The study, led by University of Florida researcher Timothy Garrett, Ph.D., devised an unbiased, well-powered and independent assessment of insulin products from major manufacturers using approved U.S. Pharmacopeia (USP) methods. (The 2017 study did not use a research method approved by the USP.) The researchers tested nine insulin formulations, purchased at four pharmacies within five geographic locations in the U.S., and the results are out, published in Diabetes Care. All of the insulins purchased from U.S. pharmacies contained the expected quantity of active insulin. Can we all say, “Phew!”
This study brings welcomed news to the T1D community, and should ease any remaining doubt about the quality of our insulin supply.
To read more about this study, visit our press release here.