Breakthrough T1D-funded scientists have made a significant advancement in beta cell replacement research. Beta cell replacement therapies aim to provide insulin on demand from cells implanted in the body. These therapies have the potential to eliminate insulin therapy and liberate people from the burdens of managing type 1 diabetes (T1D) for months or even years at a time. Today, this can be done using islet cells from donors, but only a small fraction of people with T1D benefit.
As published in Nature Cell Biology, the scientific team from the University of California San Francisco, including the lead author on the study, Gopika Nair, Ph.D., a Breakthrough T1D postdoctoral fellow, and senior author, Matthias Hebrok, Ph.D., explain how for the first time they have introduced an inventive step in the process of making mature insulin-producing beta cells from stem cells. The resulting cells show enhanced maturation properties that make them respond to blood sugar more like human beta cells.
“We are excited about our findings and believe that generating such insulin-producing cells from stem cells will allow us and others to probe previously unexplored aspects of human beta cell function, interaction with immune cells and, of course, avenues for cell therapy,” says Hebrok.
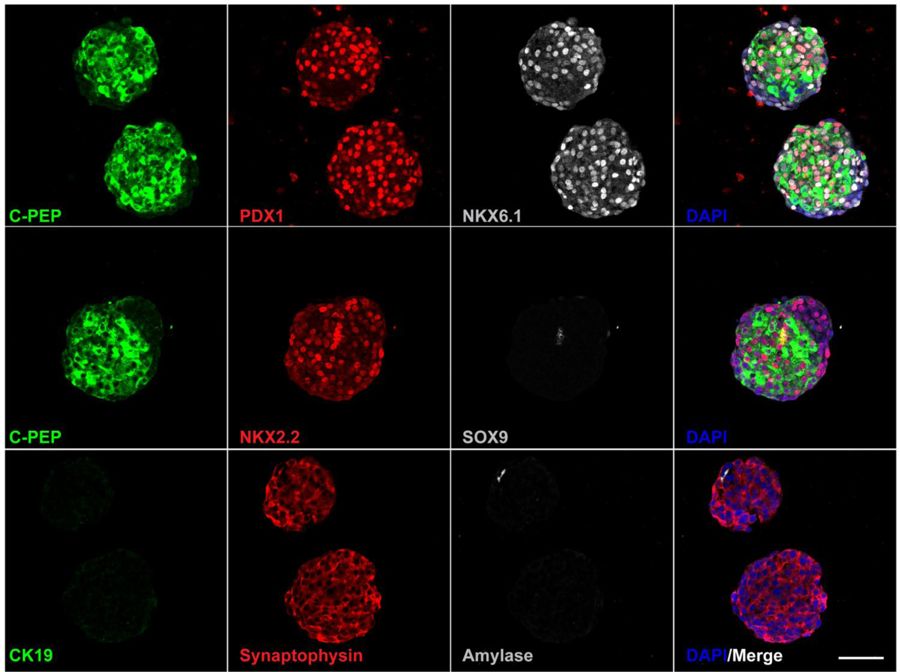
The ability to turn stem cells into beta cells has been a great achievement. Differentiation protocols to generate beta cells in the laboratory have improved significantly, and could potentially provide an unlimited source of insulin-producing cells for replacement therapy. Like in pancreatic islets, beta cells produced in the laboratory prefer to grow in clusters. However, current differentiation protocols result in a heterogeneous mixture of undifferentiated and differentiated cells, with some insulin-producing cells that remain at an immature stage.
In this study, Drs. Hebrok and Nair thought, “What if one could artificially disperse these islet-like clusters, isolate the immature beta cells, re-aggregate into enriched beta cell clusters and give them one more week in culture to further differentiate? Would they be more functional and resemble human islets than the mixed clusters?” This had never been done before. The researchers demonstrated that the enriched beta cell clusters showed an improved response to glucose, and after transplantation found that they were functional in a matter of days. (Research thus far has insulin-producing beta cells responding to blood sugar challenges only 2 to 6 weeks after transplantation.)
What’s in the future? While the methodology used to isolate the beta cells may pose a limitation for the attempt to use it in a clinical setting, the study introduces a novel and clear concept to advance the field of stem cell-derived insulin-producing beta cells. Identifying alternative methods to isolate the beta cells may allow broader applications, representing a step forward toward a time when everyone with T1D would benefit from a beta cell replacement therapy.
“This is an exciting period; not only does our discovery set the stage for cell therapy, since we can now make human beta cells in the dish, it opens avenues for finding novel drugs that can stimulate expansion of beta cells. This would be a breakthrough for people with T1D with residual beta cell mass,” says Nair. “I’m also immensely grateful to Breakthrough T1D for funding my postdoctoral research and providing me with the resources to contribute to their mission to cure T1D.”
The Breakthrough T1D-funded investigators on the publication, now or in the past, include Drs. Hebrok and Nair, and Anil Bhushan, Ph.D., Jose Oberholzer, M.D., Sapna Puri, Ph.D., and Holger A. Russ, Ph.D.
Breakthrough T1D-funded researchers are working hard to establish the most effective ways to create abundant insulin-producing cells in the lab. For more information on Breakthrough T1D’s Beta Cell Replacement program, go here.