While we look back on 2024, we can reflect upon the incredible progress we’ve made in advancing breakthroughs toward cures and improving everyday life with T1D.
This wouldn’t have been possible without each and every one of you and your continued support of our mission as we drive toward cures for T1D.
Here are the top 11 advances that together, we made happen in 2024:
Breakthrough T1D announced the launch of Project ACT, an initiative aimed at accelerating breakthroughs in T1D cell replacement therapies that do not require broad immunosuppression. Recent advances, such as Vertex’s stem cell-derived islets, have been made possible by Breakthrough T1D’s Cell Therapies program as part of our drive toward cures. The goal of Project ACT is to push research, development, regulatory policies, access, and adoption to increase the rate at which cell therapies without the need for broad immunosuppressants will become available to people with T1D.
Why this matters: Immunosuppressive drugs are a barrier to access to cell replacement therapies because of their toxic side effects, which is why islet transplants are currently only available to people with severe low blood sugar (hypoglycemic) unawareness and episodes. By striving toward a future where we realize the benefits of cell replacement therapies without the downsides of the current regimen of immunosuppressants, we will make islet replacement therapies broadly accessible to the T1D community.
Vertex’s clinical trial of VX-880, a first-generation stem cell-derived islet replacement therapy for people with severe hypoglycemia (requiring the use of immunosuppressants), has transitioned into a phase 1/2/3, or pivotal, trial. This news comes after Vertex shared incredibly promising data in the earlier phases of the trial, including 11 of 12 participants reducing or eliminating the need for external insulin.
The upcoming trial will expand to 50 people who will get a single, target dose of VX-880. The primary endpoint will be insulin therapy independence without severe hypoglycemic events after one year. This is the final clinical testing stage before Vertex can seek FDA approval.
Breakthrough T1D has a decades-long relationship with Vertex and the leading scientists behind stem cell-derived islet therapies, an advancement that would not have been possible without Breakthrough T1D funding and support. The T1D Fund had invested in Semma Therapeutics, which was acquired by Vertex Pharmaceuticals in 2019, eventually leading to the active clinical development of VX-880 in T1D.
Why this matters: This is the first time a scalable cure for T1D is entering phase 3 clinical trials—a significant win and a huge step toward accelerating the delivery of cell therapies to members of the T1D community!
Tegoprubart: Transplant Survival Without Standard Immunosuppressive Drugs
Tegoprubart, an anti-CD40L immunotherapy that limits the immune response, is being tested in a Breakthrough T1D-funded study in people with T1D and severe hypoglycemia who have received deceased donor islets. Eledon Pharmaceuticals announced promising initial results in which two of three people achieved insulin therapy independence. According to the study, tegoprubart is safer for both people and transplanted cells in comparison to broad immunosuppressants, with milder side effects and greater islet survival. To further support this effort, the T1D Fund: A Breakthrough T1D Venture invested in Eledon.
Cell Pouch: A Home for Transplanted Islets
Breakthrough T1D has been supporting the development of Cell Pouch, an implantable device from Sernova that provides a safe, immune-protected environment for transplanted islet cells. In phase 1/2 clinical trials, all six people who received donor islets within the Cell Pouch achieved sustained insulin therapy independence with immunosuppressants, including long-term islet survival and function over five years without harmful side effects.
Why this matters: Standard of care immunosuppressive drugs that help avoid transplant rejection come with unwelcome side effects, such as increased risk of infection and malignancy and toxicity to kidneys, nerves, and islet cells themselves. Breakthrough T1D is focused on finding alternative ways to keep transplanted islet cells alive and healthy so that cell replacement therapies can become more tolerable and accessible.
In a major effort spearheaded by Breakthrough T1D, the first internationally recognized clinical guidelines for those who test positive for T1D autoantibodies have been published. These include guidance on monitoring frequency, education, and psychosocial support in addition to recommended actions for healthcare professionals (HCPs) when the risk of T1D progression is high. The guidelines were cooperatively developed with over 60 international experts spanning ten countries.
Why this matters: Previously, there had been no consensus on monitoring guidelines for people who test positive for T1D autoantibodies. Standardization of clinical recommendations means that individuals, families, and HCPs have tangible next steps to monitor early T1D progression and catch life-threatening complications sooner.
- Breakthrough T1D is leading a campaign to secure a recommendation for T1D screening from the U.S. Preventative Services Task Force (USPSTF), the main authority for preventative care. Approval would require T1D screening to be covered by insurance—an important step forward in expanding access.
- Driven by Breakthrough T1D’s advocacy efforts, The Centers for Medicare and Medicaid Services (CMS) established a unique ICD-10 code for stage 2 T1D. ICD-10 codes are used by HCPs to classify and document diagnoses, symptoms, and procedures. These codes provide a unified way for doctors and providers to indicate what diseases or conditions a person has in their electronic health record (EHR), empowering HCPs to document accurate diagnoses and provide the best possible care.
Why this matters: T1D early detection is critically important to prevent life-threatening complications at diagnosis and to give people necessary resources to make informed decisions about their health. Integrating T1D screening into the U.S. healthcare system will increase access to care.
The past year has seen some important advances in glucose management therapies and devices:
- Cadisegliatin, an activator of a blood sugar regulator in the liver, is being investigated in a phase 3 clinical trial (TTP399) as an adjunct therapy to insulin for people with T1D, although it is currently placed on clinical hold. vTv Therapeutics, the trial sponsor, is also a T1D portfolio company.
- The Omnipod 5 app is now available for the iPhone, making it easier to control the Omnipod without the need to carry a controller. It can also integrate with the Dexcom G6 continuous glucose monitor (CGM).
- Eversense 365 is the first FDA-approved year-round sensor that can easily integrate with automated insulin delivery (AID) systems. Other sensors require replacement after 10-14 days.
Why this matters: While advancements in glucose management have been pivotal in improving health outcomes for people with T1D, access remains a challenge. AID systems are globally underutilized, and not everyone has the necessary technology to connect devices. Breakthrough T1D is working to not only support advances in glucose management but also increase access.
Related content: While Breakthrough T1D consistently strives to improve the lives of those living with T1D, as an organization we have made incredible progress in the development of AID systems, also called the artificial pancreas systems. Read a historical perspective written by Breakthrough T1D volunteer Doug Lowenstein that covers conception to FDA approval of the first artificial pancreas systems, which changed the lives of people with T1D.
An inquiry spearheaded by the Breakthrough T1D affiliates in the U.K. uncovered risks of developing T1D eating disorders (T1DE), including bulimia, anorexia, or insulin restriction to lose weight. There is a significant gap in education and clinical guidelines for HCPs, a lack of internationally recognized criteria for T1DE diagnosis, and insufficient care integration, leading to preventable complications and healthy years of life lost. Breakthrough T1D recognizes the importance of spreading awareness and support for T1DE, and much work is needed to improve the lives of those living with T1DE.
Why this matters: There is an urgent need to change the way T1DE is approached, including integrated physical care with mental health services to get people with T1DE the access to care that they need.
In a study that included people with T1D, finerenone (Kerendia®) has been shown to improve cardiovascular outcomes in adults with heart failure. The drug is already approved in the U.S. to treat kidney and cardiovascular disease in people with T2D. Based on these results, Breakthrough T1D is supporting a clinical trial (FINE-ONE) in conjunction with Bayer to investigate the use of finerenone for T1D with the hopes of reducing kidney complications.
Why this matters: Kidney and cardiovascular disease remain significant challenges for those with T1D, especially given the FDA’s recent rejection of an SGLT inhibitor to lower blood glucose in people with T1D and chronic kidney disease. Yet, a new clinical trial (SUGARNSALT) will better assess the benefits versus risks.
Breakthrough T1D is advocating for the regulatory approval of C-peptide, a biomarker for insulin production by beta cells, to be used as an endpoint in clinical trials. An endpoint can accurately predict a meaningful benefit in clinical trials for disease-modifying therapies (DMTs; treatments that can slow, halt, or reverse T1D). To support this endeavor, Breakthrough T1D scientists and an expert consensus panel published research with evidence supporting C-peptide as an endpoint. Breakthrough T1D is continuing to engage with regulators, coordinate with industry, and assess more clinical trial data to drive this effort forward.
Why this matters: Current clinical trial endpoints (HbA1c, hypoglycemia, and complications) are not the best way to gauge the clinical benefits of T1D therapies. If C-peptide gets regulatory approval to be used as an endpoint, clinical trials could be smaller and shorter while still accurately assessing the advantages of a DMT. This means that drug development can move more quickly, and people with T1D will be able to access therapies sooner.
Related content: Two years ago, the T1D community received the incredible news that Tzield® had become the first FDA-approved disease-modifying therapy that can significantly delay T1D onset. Breakthrough T1D volunteer Doug Lowenstein recounts the life-changing drug’s journey nearly 100 years after the discovery of insulin.
The T1D Index is a data simulation tool that measures the global health impact of T1D, bridging gaps in our knowledge of public health statistics. T1D Index 2.0 has new and improved functionality, including advanced simulation capabilities, validation of data, and enhanced user experience. Breakthrough T1D contributed to both the development and improvement of the T1D Index.
Why this matters: The T1D index is critical in defining the intercontinental scope of T1D, driving us toward country-specific solutions and improved global health outcomes.
Earlier this year, JDRF rebranded to Breakthrough T1D. While our mission remains the same, our name needs to better reflect who we are and where we’re going. Our new brand aligns with our mission to accelerate life-changing breakthroughs for those of every age living with T1D as we work toward a world without it.
Why this matters: The proof is in the name—each day we strive to increase and accelerate breakthroughs in T1D, and it’s critical for our brand to accurately reflect our mission.
It’s certainly been an exciting year! While we still have more work to do, it’s crucial to celebrate our wins, both big and small, to see how far we’ve come in our push to make T1D a thing of the past.
Together, we’re accelerating breakthroughs for people with T1D, and the support of the T1D community drives our mission forward every single day, leading the way to lifechanging therapies and cures. Let’s see what 2025 has in store!
Table of Contents
Author’s Note
Chapter 1: The Beginning: A Parent
Chapter 2: The Scientist
Chapter 3: The Patient Organization: Cure or Treat
Chapter 4: “Now What Do We Do?”
Chapter 5: The Big Gamble
Chapter 6: The Device Makers
Chapter 7: The Inventor
Chapter 8: The Tide Turns
Chapter 9: The Allies
Chapter 10: The Regulators
Chapter 11: The Hackers
Chapter 12: The Patients
Chapter 13: The Finish Line
Chapter 14: The Aftermath
Postscript
Author’s Note
In April 2001 our younger daughter Emma was diagnosed with T1D at age 14. We quickly found our way to JDRF and dove into working with our local chapter in Washington, DC. We were inspired when we heard there would be a cure in five years. We got excited about islet cell transplants only to realize that they would never be of use to Emma. We got excited about every trial that cured T1D in mice. But as the years dragged on, we watched and helped Emma struggle to manage T1D and we ached for her. We started to drift away from JDRF because we no longer believed that JDRF’s singular focus on a cure would help Emma. What was it doing to keep her healthy until a cure was found? Along the way, we met kindred and similarly frustrated and impatient spirits like Jeffrey Brewer. We heard this young new JDRF scientist Aaron Kowalski speak passionately about a new initiative he was leading called the Artificial Pancreas Project. And we were inspired anew.
In 2023, I wrote a story about the development of the first-ever T1D disease-modifying immune therapy, teplizumab. When I finished, I started thinking about other stories in the T1D field that deserved to be told. I quickly focused on the history of artificial pancreas development as a possibility. I realized that while others have written about it, the definitive history of this remarkable journey had yet to be written. In the pages that follow, I try to fill that void.
While this story focuses on a handful of people who played central roles in securing approval of AP systems, space does not allow me to highlight the instrumental contributions of hundreds of passionate scientists, Breakthrough T1D volunteers, clinical trial participants, industry executives and researchers, regulators, lawmakers, and government scientific agencies. While they may not receive the explicit recognition they deserve, what follows is their story as much as it is the story of those featured. They all helped make history, and helped people like my daughter live safer and healthier lives. Our family owes them a debt of gratitude we can never repay.
This story is based on more than 30 one-on-one interviews lasting a total of more than 40 hours, and review of hundreds of pages of peer-reviewed articles in scientific journals. I am responsible for the content of this story in its entirety. I hope you enjoy reading it.
Douglas Lowenstein
Washington, DC
November, 2024
Chapter 1: The Beginning: A Parent
It was 2002 and Jeffrey Brewer, a successful technology and Internet entrepreneur, was worried and frustrated. His son Sean had been recently diagnosed with type 1 diabetes (T1D). T1D is a burdensome autoimmune disease where a person’s immune system mistakenly destroys the insulin-producing beta cells that live in the pancreas.
“The doctors explained to me Sean would be dependent on a hormone called insulin,” Brewer recalled. “They told me the dangers of not dosing the drug correctly: too little and his blood sugar (or blood glucose) levels would be too high putting him at increased risk for long-term health complications; too much and he was at risk for low blood sugar with the potential of becoming unconscious and even dying if no one’s around to help. I was given some needles, insulin vials, a blood glucose meter1, and literally a handwritten sliding scale to calculate correct insulin doses throughout his day. I couldn’t understand why it was so rudimentary.”
Much of the food we eat contains carbohydrates, a form of sugar, or glucose, that our body converts to fuel. In healthy people, when the body absorbs sugar from carbs, it makes just the right amount of insulin to ensure that blood-sugar levels remain safe. But those with T1D are stripped of their capacity to make insulin, so they must execute a delicate dance where the amount of carbs they consume is precisely balanced by the amount of insulin they dose. Over a typical day, the individual with T1D, or their caregivers, must make dozens of complex calculations daily where a single mistake could be fatal. With the technology at hand when Sean was diagnosed, the task was virtually impossible to get it right for a day, let alone for weeks at a time.
Tall and lanky, Brewer was almost always dressed in khaki slacks, a button-down shirt with a school backpack over his shoulders. He was intense and restless, and a friend said, “you can always hear his mind clicking.” These characteristics explain why he was convinced there had to be a better way to manage T1D. And soon he gravitated to an organization he believed would help his son and others safely manage their disease.
The Juvenile Diabetes Research Foundation (JDRF)2 was a nonprofit founded in the 1970s by a group of parents dedicated to funding research to cure T1D. By the early 2000s, JDRF had poured money toward that goal over a 30-year period and while there were some promising advances no biological cure was in sight3.
As Brewer got more involved, he joined a committee of JDRF volunteers that reviewed grant applications brought forward by the research staff, where he had a chance to interact directly with leading T1D scientists. He and other committee members heard one proposal after another to carry out basic science experiments rather than projects that would directly lead to therapies helping people already living with T1D. His doubts about JDRF’s priorities began to grow.
“I realized very quickly that we weren’t anywhere close to a cure for T1D,” Brewer recalled. “In the meantime, my son was going to need something better than what was commercially available for a long time.” With this realization, Brewer had his mission—to keep Sean and others with T1D healthy so that when a cure arrived, they would be healthy enough to benefit. He also had the tenacity and business experience to pursue this goal.
[1] Blood glucose meters are handheld devices where the user pricks their fingertip with a needle that draws a small spot of blood that is then put on a strip and inserted into the machine to generate a glucose reading. The constant pricking can create scars and can be mildly painful.
[2] The organization was rebranded as JDRF in 2010 and in 2024 was again rebranded as Breakthrough T1D.
[3] Around this time, Canadian Dr. James Shapiro at the Alberta Diabetes Institute pioneered islet cell transplants, a procedure to harvest pancreatic cadavers, remove the islet cells where beta cells lived, and transplant them in people with acute T1D. Some people felt this was a major step toward a cure but numerous practical issues, including a limited supply of pancreatic cadavers and the need for recipients to take a lifetime of immune suppressive drugs with adverse side effects, limited its potential for most people with T1D.
Chapter 2: The Scientist
Aaron Kowalski was also frustrated.
Kowalski’s younger brother Steve had been diagnosed with T1D in 1977, and a few years later, in eighth grade, Aaron was diagnosed. “There was a lady who lived on our street fully blinded by T1D,” he recalled. “When we first met her, she had a cane and then she had a seeing eye dog. She was in her twenties. When I was diagnosed, I was like, ‘I just don’t want to go blind.’” Like Brewer, Kowalski was certain there had to be a better way to manage T1D and in his third year of undergraduate work at Rutgers University, he decided to become a biology major “with the purpose of going to graduate school to work in diabetes.” He would go on to earn his Ph.D in microbiology and molecular genetics, anticipating a career in the pharma industry.
He started working at a hospital in Newark, NJ, doing research on hypoglycemia (low blood sugar). But he wasn’t happy. “I was looking for post-doctorate jobs in various scientific journals and I saw an ad from JDRF seeking a scientific project manager to work on diabetes complications including hypoglycemia, exactly what I was doing. I literally typed up an email and sent it.”
In September 2004, JDRF hired him as a research scientist to lead its program to find ways to mitigate the long-term complications of T1D ranging from blindness to kidney and cardiovascular disease to neuropathy.
Kowalski knew that a landmark study (the Diabetes Control and Complications Trial, or DCCT) by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), a unit of the National Institutes of Health (NIH), had found that people with T1D could reduce the risk of long-term complications by 35-76% through extremely tight blood-sugar control—keeping their levels in a range that largely eliminated dangerous highs and lows. There was just one gigantic problem: achieving the DCCT ideal target blood-sugar levels was virtually impossible to do safely for even the most meticulous person living with T1D using the existing “rudimentary” technology.
“The people in the DCCT who had intensive management had an incredible amount of help during the trial 24/7,” said Judy Fradkin, who would eventually become director at NIDDK’s diabetes division. “Even knowing the huge impact it had on them during the seven-year trial, they absolutely couldn’t maintain the level they had in the DCCT after it ended.”
Kowalski projects energy and optimism. He is articulate, passionate, and determined, and his mind, like Brewer’s races a mile a minute. These traits would eventually lead him to become JDRF’s current CEO (Brewer served as JDRF CEO from mid-2010 to mid-2014). But back then, he was at the bottom of the JDRF scientific food chain and one of his first assignments was to attend a conference of the Diabetes Technology Society in Philadelphia. Prior to the main conference session, Kowalski was stunned to meet a group of scientists and patients working with a device that would continuously monitor blood sugars 24/7, a result that blood glucose meters could not match unless a person pricked their finger a few hundred times a day.
“Continuous glucose monitoring (CGM) was a holy grail,” Kowalski recalled of his encounter with the scientists. “I go to this meeting, and they have a CGM and they’re testing it. It alarms when your blood sugars are low or high, you see your blood sugar number all day long.’ It was the greatest news I’d ever heard. It was amazing.” And it offered the hope of achieving the tight controls recommended by DCCT and adopted by clinical organizations like the American Diabetes Association.
The next day, Kowalski attended the plenary session of the conference, and his mind was still buzzing with excitement about the CGM he had seen the day before. The first speaker was a renown T1D endocrinologist who declared that people with T1D can do just fine using the current tools of glucose meters, injections, and insulin pumps. When the Q&A began, someone in the room rose and said, “Doctor, I give you a lot of credit to stand up in front of a room like this and to say something so incredibly stupid and outrageous. That is the dumbest thing I’ve ever heard.”
The mystery man was Jeffrey Brewer and the notion that the available tools were adequate enraged him. Soon after the event, Brewer and Kowalski talked for the first time. Kowalski later said they were “peas in a pod” with a shared determination to improve management of T1D. Their objective was to persuade JDRF to commit research dollars to development of an artificial pancreas system (APS)4 – a system where a CGM would “talk” in real time to an insulin pump and direct it to dose exactly the right amount of insulin to safely control blood-glucose levels with limited intervention by the user. In short, the idea was to come as close as humanly possible to mimicking the function of the human pancreas by automating insulin delivery and relieving people with T1D from the burden of counting carbs, dosing insulin, and making life and death decisions based on gut and math 24/7.
[4] The term artificial pancreas has become common to describe the systems that were eventually developed. They are also referred to as Automated Insulin Delivery Systems. However, technically, the current devices do not mimic a real pancreas for several reasons, including the fact that the pancreas has other hormones involved in blood sugar regulation besides insulin, and they still require some user intervention. Regardless, in the period covered by this story, the term “artificial pancreas” was commonly used and thus that is the case here.
Chapter 3: The Patient Organization: Cure or Treat
An artificial pancreas was not a new idea. In fact, Dr. Ernst Freidrich Pfeiffer developed a machine called the Biostator in 1974, an insulin pump with intravenous (IV) continuous glucose monitoring and IV insulin infusion. The machine was the size of a refrigerator and was only feasible for hospital settings. Thirty years later, the idea of creating a wearable, user-friendly artificial pancreas remained mostly an idea, perhaps dabbled with by some scientists around the world but not of interest to diabetes technology companies or many others. In fact, a few years before the Brewer-Kowalski team formed, JDRF itself had set an organizational goal to develop an artificial pancreas. But it had not allocated any funds toward this goal mainly because senior staff and volunteer Board leadership believed passionately that diverting resources from curing T1D would break faith with donors and throw the organization off course from its singular mission and identity to cure T1D.
John Brady was a member of the JDRF Executive Committee at the time. His son Phillip had T1D. “The intensity and passion of those who had been involved for years was incredibly high. They did not see room for treatment in our mission. They argued we were founded to find a cure, not to treat the disease.”
Brady disagreed and quickly emerged as a key ally of Brewer and Kowalski. “What Jeffrey wanted made complete sense to me—to keep people alive and healthy until we find a cure. We were losing people, kids dying in bed overnight of low blood sugars. If we could automatically dose insulin and have everybody go to sleep and all wake up, that was an incredible victory.”
Brewer and Brady, along with a handful of other volunteers, worked to educate and persuade other key volunteer leaders. Kowalski was working the inside, trying to persuade his research staff superiors to go all-in on funding development of AP systems. He recalled that his bosses told him to stop ‘stirring things up.’ I said I didn’t care. We have got to do this.”
Early in 2005, and now a member of the JDRF International Board, Brewer “decided to force the issue of the artificial pancreas with the JDRF board in the following way: I made a directed gift of a million dollars that was contingent upon that money going to fund proof of concept research for integration of CGM and insulin pumps and development of algorithms for automated insulin dosing.”
Brewer remembered that “they said my gift will dilute the mission, and industry was already taking care of it. But they knew they didn’t want to turn away the money.”
Looking back at the Board meeting, Brady remembered the drama and tension. “It was not a slam dunk. It was probably the most emotional debate that I ever witnessed in all my time on the board. And it was far from unanimous. There was a significant portion of the board who thought it was wrong. This was not something that had strong widespread support, both as a project, nor as a redefinition of our mission to include treat.”
Kowalski recalled that some of the most influential Board members opposed accepting Brewer’s donation. “Some were vehemently against it. One of them was on the Board of Johnson & Johnson and he said ‘J&J’s research budget for diabetes is hundreds of millions of dollars. You’ll never make an impact.’ It was a huge throw down. And Rob German, the chairman, said, ‘this guy’s passionate, he’s willing to put his money up. Why don’t we get somebody on the board, set them up with Jeffrey and this Kowalski kid and give them six months to convince us you can spend a million bucks.’” Said Brewer: “They didn’t know partnering me with Aaron was basically putting the fox in the hen house.”
The study commission was led by highly respected Board member Charles Queenan. In October 2005, Queenan presented the study commission recommendations including “to pursue [an artificial pancreas] with the same intensity as other JDRF cure therapeutics goals.” According to Board minutes, the Board agreed to approve the recommendations.
With this, the Artificial Pancreas Project (APP) lifted off. It marked a turning point in JDRF’s research priorities as the organization fully embraced the commitment to keeping people healthy on the path toward a cure. Within a year, the emotional battles over cure vs treat would be largely forgotten. The JDRF commitment would help catalyze the most significant advance in T1D treatment in nearly a century. Between 2005 and 2024, JDRF would spend $171 million on artificial pancreas related research that would result in the commercialization of a series of systems that partially automate insulin delivery and dramatically reduce the daily burden of T1D while improving the daily and long-term health of people with the disease.
Chapter 4: “Now What Do We Do?”
There is a scene in the movie The Candidate where Robert Redford’s character, Bill McKay, has learned he was the upset winner for a U.S. Senate seat in California. Just before McKay goes down to speak to his supporters, he turns to his chief advisor and asks, “What do we do now?”
That was the exact question facing the small team at JDRF charged with creating an artificial pancreas. The diligence study had made one thing clear: this would not be your mother’s science project. It would have to run on multiple parallel tracks: the science and engineering track—developing algorithms—the mathematical formulas that would track data from the CGM and adjust insulin dosing minute by minute to keep blood sugars in a safe range; the regulatory track to understand what the FDA would require to grant approval of an artificial pancreas; the industry track to encourage investment and commercialization of AP systems; and the reimbursement track to persuade private and public health plans to cover the system upon approval.
It was unlike anything JDRF had ever undertaken. And it would need someone with a unique set of cross-sectional skills to lead. JDRF CEO Peter Van Etten had just the guy: Larry Soler, JDRF’s Washington, DC-based chief lobbyist who had organized and led a multi-faceted advocacy campaign to overturn bans on stem cell research and JDRF’s advocacy campaigns to ensure multiple renewals of the Special Diabetes Program (SDP) that provided NIH with hundreds of millions of dollars for T1D research since its original enactment in 1998. To Soler, his work was more than a job. He himself had been diagnosed with T1D in his early 20s.
And Soler had exactly the right partner in a recent hire who had unexpected bandwidth due to congressional paralysis on an unrelated issue. Her name was Cynthia Rice, a Harvard graduate with experience as a Senate staffer, working in nonprofits, and a stint staffing President Clinton’s Domestic Policy Council specializing in health policy. Soler and Rice were both preternaturally calm. Both were highly meticulous and organized, and both knew their way around the corridors of power in Washington, DC, which would be an epicenter of activity given the presence of the U.S. Food and Administration (FDA), the regulatory body that would have to approve for commercial use in the United States any artificial pancreas system. (Soler would leave JDRF in 2009 to become the first CEO of Michelle Obama’s anti-obesity nonprofit, Partnership for a Healthier America, and Rice would take over the lead role, seeing the project to its completion before she retired in 2023).
As the team organized and started building out a strategic plan in December 2005, the NIH hosted the first of what would become a series of key research and interagency conferences on artificial pancreas technology over the next decade. The focus was on exactly how to pursue the goal. It turned out that not everyone there felt the goal was attainable.
One of the attendees was a University of Virginia Ph.D. mathematician named Boris Kovatchev. Kovatchev’s father had been diagnosed with insulin-dependent diabetes at the age of 48 and it made a huge impression on him. “He had a couple of toes amputated. It was a really bad case.” Kovatchev especially grasped just how difficult it was for people with the disease to correctly dose insulin, and upon arrival at UVA in the 1990s, he quickly started specializing in T1D, including tinkering with algorithms to manage insulin dosing.
“There were debates at the NIH meeting whether it is even possible to do an artificial pancreas,” he recalled. “There was a prominent scientist who said it is not possible. There were essentially two camps, one was ‘let’s try it,’ and the other was ‘it’s not possible.’” Said Kowalski: “This prominent researcher stood up and said ‘you’re going to kill people.’” But despite some skeptical voices, Kowalski told the gathering that JDRF would move ahead and seek applications to fund key research to advance the project, especially in the area of developing the critical algorithms that would be the brains of the operation.
By August 2006, teams of researchers at multiple academic centers had received grants of about $300,000 each to take on various aspects of AP development. Some had dabbled in the field for years, others more recently. But collectively they were a Murderers’ Row of T1D scientists. Bruce Buckingham at Stanford, Bill Tamborlane and Stu Weinzimer at Yale, Kovatchev at UVA, Roman Hovorka at the Wellcome Trust-MRC Institute of Metabolic Science at the University of Cambridge in the UK, and Frank Doyle, Howard Zisser, and Eyal Dassau at University of California at Santa Barbara (UCSB)5 were among the grant recipients.
Another early grantee was the Jaeb Center for Health Research, founded by Roy Beck. His organization’s role wasn’t the headline-grabbing work of conducting high-profile trials, but it was a critical component of the project. For the next decade, Jaeb coordinated virtually every important clinical trial, engaged with the FDA on trial design, captured and analyzed data from the trial, and supported the publication of the research in peer-reviewed scientific journals. It also served as secretariat for the JDRF-created Artificial Pancreas Consortium, a series of meetings over the years bringing virtually every major researcher, funder, and company in the field together to exchange information and ideas. “What Jaeb did was herd some of the wildest, cockiest people,” said Kowalski. “They helped us enforce the rules of the consortium so people weren’t going off on their own screwing things up. Roy is probably one of the most important people in diabetes that nobody’s ever heard of.”
While researchers were energized, two immediate challenges emerged.
One was the U.S. Food and Drug Administration (FDA), the regulatory agency that reviews and must approve for commercial use the safety and efficacy of all medical devices and drugs. In a meeting in the early months of the project, JDRF’s team met with FDA officials to explore what data and studies the agency would want to see as a basis for reviewing AP systems. The response was discouraging.
“We went into their conference room and, boy, did they shut us down,” recalled Soler. “They basically told us that if we are thinking about testing these systems in humans we should instead be talking about testing in mice. I remember leaving and feeling, whoa, this went really bad.”6
The second concern was the reliability of what would be the nerve center of the AP system—the continuous glucose monitors that Kowalski was so enthused about months earlier. FDA had approved early versions of CGMs in the late 1990s, but they did not actually perform real-time blood-sugar monitoring. In 2006, it approved two newer “real-time” systems developed by the major device maker Medtronic, and a new start-up named Dexcom. But the FDA only allowed these CGMs to be used as a secondary measure of blood-glucose checking because it still felt that the finger prick meters were more reliable.
“FDA didn’t allow the use of CGM data for decision making and could not reconcile that insulin would be dosed automatically based on a reading from a device they deemed inaccurate,” said Sanjoy Dutta, currently chief scientific officer and head of Research at Breakthrough T1D (formerly JDRF) who joined the JDRF APP team a few years after the project started. “The initial CGMs had high levels of inaccuracy,” said Dutta. “They were the Achilles Heel of developing AP systems.”
FDA’s concerns about CGMs were troubling enough without the fact that health insurers declined to cover them (they could cost up to $5,000) because they were not persuaded that they were more accurate than existing glucose meters, a view reinforced by the FDA’s decision to approve them only as secondary measures. If insurers would not cover CGMs, it was even less likely they would cover even more advanced and complicated AP systems, and without some certainty around coverage the prospect for AP development would be dim.
[5] Each of these researchers were part of larger teams that participated in the groundbreaking work, but it is impossible to list all the names here.
[6] Typically, research includes a preclinical phase where drugs and devices are tested in animals, often mice. But these studies are costly and lengthy and would set the AP project back years before it got off the ground.
Chapter 5: The Big Gamble
“We realized that if the sensors weren’t successful, we couldn’t get to a closed loop,” said Kowalski. “And nobody believed in the sensors. They all sucked. And we went around and asked ourselves what we need to do” to prove that even less precise early sensors would still be better for patients than glucose meters. The discussions led JDRF to take a big gamble and invest $10 million to conduct a yearlong, multi-center trial of the new sensors now being developed by Medtronic, Dexcom, and a third player, Abbott, to prove to insurers that CGM were more accurate than glucose meters.
“We made all the decisions about this trial, but we consulted with the companies,” Rice said about the team’s meetings and calls with device makers and health insurers. “The companies were very risk averse.” But ultimately, working with Jaeb, and believing they could design a sound study that would prove the latest CGMs were superior to any existing blood meters, JDRF moved forward. The trial that would be historic for its folly or brilliance launched in September 2006.
Kowalski remembers a meeting with Claudia Graham, a senior executive at Medtronic. “She said to me ‘you guys could screw up this whole field if the trial fails; this whole field will be dead.’” Graham remembers the conversation. Her comment was rooted in her concern that the first generation CGMs were “kludgy” and finicky and hard to use for many. Concern about doing the CGM study) was that these were all first-generation devices. “I was very worried the results would reflect those imperfections at the time.”
JDRF’s Soler said: “The trial was a huge gamble. It was hugely expensive, and the goal was to show that people who use CGMs do better than people who don’t.”
If that goal was not achieved, Graham’s fear would be reality.
Chapter 6: The Device Makers
Al Mann was the son of a Portland, OR grocer and a mother who was a pianist. Mann’s brother was a violinist who became a founder of the famed Juilliard School of Music, and his sister was a concert pianist. But Al went another direction, getting a degree in physics and becoming a serial entrepreneur, starting companies in fields ranging from aerospace to cardiac pacemakers. Eventually, in the late 1970s, he turned to diabetes, starting a company called MiniMed which developed insulin pumps. In 2001, he sold MiniMed for $3.7 billion to one of the country’s leading medical device makers, Medtronic. By 2005, Minneapolis-based Medtronic was the undisputed giant in the T1D device space. Al Mann remained involved, and he was the person who flatly stated that at the 2005 NIH meeting that AP systems would “kill” people.
To JDRF, engaging industry was a critical objective because products are not developed and sold by academic scientists working in labs. Indeed, a foundational tenet of the AP Project was to catalyze industry involvement. In fact, in one of Brewer’s presentations to the JDRF Board, he had a slide which simply said: “Without JDRF, still ten years away.” So, JDRF made it a priority to attract Medtronic to AP development. But the company was not taking the bait.
“They were sitting fat and happy owning the insulin pump business,” said Brewer. “We went out to their headquarters in Minnesota for a big dog and pony show. I remember they had an iceberg slide which showed nothing at the top, but they told us what’s going on with AP was confidential and below the surface and that we didn’t have to worry about it, and we should go back to funding the cure. But we knew they in fact they had few people working on it under a small NIH grant and they weren’t putting a dollar of their own capital into the AP. They didn’t see the need to do so. It wasn’t going to help them expand the pump market which they already owned.”
“The problem with Medtronic was there was a certain degree of arrogance,” said Graham who retired from Medtronic in 2008 (she eventually joined Dexcom. “They thought it was all about the pump. They had hundreds of thousands of pumps in the field with four-year warranties so for them to easily shift over [to AP systems] was a big business hurdle.”
Medtronic’s seeming slow pace created a new imperative. Abbott, Dexcom, and Johnson & Johnson’s diabetes subsidiary Animas were much smaller players and fighting to gain a foothold in the CGM market. Leaping directly to AP development was seen as high risk.
“For JDRF the fundamental problem we had was to create competition where there was no meaningful competition in the pump market,” recalled Brady. “Medtronic was the gorilla and had the ability of doing it, the technology, and the resources but there was no competitive market incentive for them to move forward.”
Chapter 7: The Inventor
When Dean Kamen was in his teens growing up in Long Island, NY in the mid 1960s he started making and selling to local bands homemade lighting control systems. Around this time, his older brother Barton had earned a Ph.D. in pharmacology and was focused on pediatric oncology.
“My brother would come home and explain what he was doing in neonatal ICU. He had some ideas on how to do cancer treatments better in babies. He wanted something that could give them very small amounts of drugs and to time the dose in intervals instead of running on an IV drip.” Kamen developed a pump device that met his brother’s specifications, using off-the-shelf components including the base of a standard butter dish. He made the units in his parent’s basement and drove them to his brother at Yale. “It wasn’t a business,” he recalled. “I was just trying to help my brother save babies.”
But the brotherly collaboration would change diabetes history. A few years later, Dean Kamen was at Worcester Polytechnic Institute. His brother Barton showed the pump device to a colleague at Yale. The colleague was the same Bill Tamborlane, who would co-lead some of the most seminal AP trials years later. But at the time, he was working with pregnant women with T1D.
According to Kamen, Tamborlane envisioned adapting the pump system to dose insulin. He imagined a pump that would provide a steady baseline, or basal, dose of insulin through the day, and allow the user to give a higher, or bolus, dose at mealtimes to account for the carbohydrates consumed. Kamen took on this challenge and developed a wearable pump weighing 17 oz. It was the first insulin pump in history. It was known as the “blue brick” because this time Kamen could only find blue butter dishes. Soon, Kamen created his first company, Auto Syringe, with the blue brick at its center. In 1981, the drug company Baxter International purchased Auto Syringe.

After the sale to Baxter, Kamen continued to create inventions in a range of fields, including healthcare. But he stayed abreast of the insulin delivery and CGM market, and, by 2005, his mind returned to whether he might invent a way to automate insulin delivery combining a CGM with an insulin pump. “I thought if they can make sensors that can measure blood glucose levels every couple of minutes we ought to be able to do a closed loop control so I said, ‘I got to go back and develop a technology that eliminates the syringe and its mechanical limitations, I got to make it small and light, and I’ve got to invent a measuring system that can measure down to fractions of a microliter how much insulin I am delivering every time.’”
So, it made sense that when JDRF began to explore options to develop AP systems, Kamen, by then founder and CEO of DEKA Research and Development Corporation and now world famous for his invention of the Segway, would be one of their first stops.
It soon became clear that the dream of creating competition might be realized.
“We went and toured the engineering facility, and we ended up making multiple visits up there,’ said Soler. And in the process, they learned that Kamen had been exploring with Abbott the possibility of creating an AP system pairing Abbott’s Navigator CGM, with Kamen’s new pump and dosing algorithm that his team developed. Soler recalled the team’s emotions as the dialogue continued. “You had a company that had a very promising CGM. They were building a significant insulin pump with Dean. Dean was an innovator. Dean had built the first insulin pump and has been involved for a long time and had a record of innovation. And so, we got really charged up by it, and we went for it.” “It” in this case was putting together a multi-party deal between Kamen’s DEKA R&D, Abbott, and JDRF.
“We had negotiations about the different IP rights, how much funding we would invest based on milestones, and what our royalty rates would be,” Soler recalled. “It was a long process, but by 2008 we got to the point where we had a deal that was agreed to by the parties.” As the deal wrapped up, Abbott announced that the President of its diabetes division who had been its lead negotiator would move over to become president of the cardiac division. The news did not trigger any alarm bells at JDRF.
“We scheduled an in-person meeting at the JDRF headquarters in New York to sign the final documents,” Soler said picking up the story. “It was in the evening and lower Manhattan was dark. The lights were out in every office except for the one conference room. We had the papers all laid out for signature and were waiting for the new executive in charge, Heather Mason, to arrive. But we kept getting alerted that the company plane was delayed. And finally, she contacted us directly and said she didn’t want to do the deal. So, she never showed up. We thought we would be celebrating. But she left us waiting.” The deal was dead.
Chapter 8: The Tide Turns
Despite the crushing disappointment over the Abbott deal, there was still cause for hope. For starters, the scientists JDRF funded in 2006 were delivering promising results.
In 2007, Doyle’s team at UCSB had published data showing that they had developed a closed loop system using a Dexcom CGM and an Omnipod insulin pump made by Insulet that communicated with one another using an algorithm that adjusted the insulin infusion rate every five minutes based on predicting future glucose levels. It was an important landmark in AP development.
At UVA, and across the Atlantic in Padua, Italy, Kovatchev and Claudio Cobelli, were collaborating to develop a simulator—a computer model—representing 300 different metabolic systems that allowed them to test scores of real-life scenarios, such as creating scores of different types of meals with varying carbs, different forms of exercise for different periods, and then testing multiple different algorithms to see, among other things, how much time the simulated “people” were in range, what risks emerged, and how many hypoglycemic events occurred. The idea was to prove to the FDA that these simulated models were an accurate substitute for testing artificial pancreas algorithms in humans not animals.
In January 2008 the FDA accepted the simulator as a substitute for animal testing. Three months later, Kovatchev’s team started an in-hospital human trial at UVA. Cobelli and a third colleague, Eric Renard in France, quickly followed suit. “The simulator saved at least five years of animal studies because we didn’t require an algorithm to be tested in an animal model to be deemed safe and effective before going into human studies,” said JDRF’s Dutta. “That entire chunk was eliminated thanks to Boris and Claudio.”

Also, in 2008, Weinzimer and Tamborlane at Yale published results of a first in human study of 17 adolescents using a rudimentary but nonetheless fully automated closed loop system. They reported that “closed loop glucose control using an external sensor and insulin pump provides a means to achieve near-normal glucose control in youth with T1D during the overnight period.” When the team presented their findings at a JDRF research summit, “people were literally crying,” Weinzimer recalled.
In even bigger news, in September 2008, JDRF’s big gamble to run a CGM trial paid off. The trial proved conclusively that CGMs are associated with improved blood-glucose control in adults with T1D compared to those who used standard therapy. “People still cite it as the landmark JDRF CGM study,” Kowalski said proudly. “I remember when that paper was published in the New England Journal of Medicine that it was like the whole world changed.”
The goal of the paper had been to persuade health insurers to initiate coverage of CGMs. But even before it came out storm clouds formed. One of the largest insurers, United Healthcare, was on the verge of issuing a non-CGM coverage decision. It would have sent a potentially fatal message to other payers, potentially crushing the CGM market before it was off the ground, and even taking down the artificial pancreas market still in its infancy. JDRF tried to reach out to its contacts at the company but hit a brick wall. Cue its network of well-connected volunteers.
“We learned that United Healthcare was planning a CGM non-coverage decision,” said Rice. “I put out an all-points bulletin to our network, asking if anyone had connections to senior executives at the company. Lo and behold, one of our leaders, Pam Sagan, had been friends for many years with a UHC executive and his wife, and that executive had since become CEO of the company. Pam was able to get through to him quickly and share that important new research was going to be published shortly, and it would be in UHC’s best interest to delay its non-coverage decision to reconsider the new evidence,” Rice recalled.
“The CEO got the decision delayed and a meeting for us to brief senior medical staff on the new findings. United Healthcare—the largest health plan in the country—ended up covering CGMs based on the JDRF study results and this volunteer intervention.” Soon, virtually every insurer would announce a favorable coverage decision.
But there were still some headwinds. Kowalski kept hearing grumbling that the project was going nowhere because there was no obvious commercial product. Sure, the lab research with somewhat jerry-rigged systems was nice but they were too cumbersome and complicated to actually be developed into a viable, wearable commercial device.
Kowalski knew they had a point. Industry needed line of sight into where all this would lead not happy talk and assurances from JDRF and scientists. What emerged would eventually be known simply as The Roadmap, a set of six boxes with iterative devices that suggested concrete products with escalating complexity and automation.
“When we got the CGM trial data, apart from the fact that the sensors helped people, a big ‘ah ha’ was nobody appreciated how much time people were spending with high and low blood sugars every day,” said Kowalski. In fact, the data found that if you were hitting one of the targets recommended by clinical organizations based on the DCCT data, “you were still spending almost 12 hours a day with a high blood sugar and 80 minutes with a dangerously low one. It blew people’s minds.”
This data crystalized a path forward. “I had the idea that a first product could turn off the pump when people are low” so that it didn’t continue to dose insulin and plunge them into severe hypoglycemia. The so-called low glucose suspend system would not be the ultimate goal for automated insulin delivery, but it would be less daunting to develop. And by suspending glucose when the CGM reached a specific low reading it would help overcome FDA’s concerns about rogue glucose sensors triggering a dangerous low blood-sugar event by continuing to dose insulin well past the safe level.
In the end, Kowalski created six product visions grouped into boxes. They culminated in Box Four, a “hybrid” closed loop where insulin delivery would be mostly automated except at mealtimes when the user would have to “bolus” to account for the carbs consumed (thus hybrid closed loop, not fully closed loop), Box Five where, a fully automated closed loop, and Box Six, a system that not only dosed insulin but other pancreatic hormones that impact glucose control. Published in Diabetes Technology and Therapeutics in September 2009, the paper for the first time laid out a manageable development plan for companies that made the ultimate goal seem more attainable. Looking back, Roy Beck said that Kowalski’s seminal paper “has been the roadmap for the last 15 years in artificial pancreas development and is still cited.”
And in January 2010 JDRF’s efforts to engage industry paid off. It struck a deal with Johnson & Johnson subsidiary Animas to build an AP closed loop system using a CGM provided by Dexcom. JDRF committed $8 million over three years to support the project. Recalling how important this was, Soler said: “We had a major medical device company make a public declaration that they were going to create an automated insulin delivery system, and it really galvanized the attention of the diabetes industry.” (The irony was not lost on some that in the JDRF debate whether to accept Brewer’s funding, one leading opponent had declared that J&J was never going to invest in the artificial pancreas space. Five years later, they did just that.)
Chapter 9: The Allies
David Panzirer’s daughter Morgan was diagnosed with T1D in March 2007. Immediately, his grandmother, New York real estate mogul Leona Helmsley, made a $5 million donation to JDRF and another organization in the T1D field. Upon her death five months later, her will stipulated that her assets be liquidated and transferred to a charitable trust, Helmsley Charitable Trust (HCT). Panzirer became a trustee, and together with his co-trustees they set up a series of core programs including a focus on T1D. If Morgan’s diagnosis wasn’t enough motivation, his other daughter Caroline was diagnosed in 2017. Over the years, Panzirer has developed a visible, respected, and well-cultivated image as someone willing to be outspoken and willing to disrupt the status quo if he feels it would help the lives of his kids and the entire T1D community. HCT and JDRF have partnered and collaborated on numerous transformational initiatives, with occasional friendly disagreements.
HCT would prove to be a powerful ally, lending its voice to the effort to educate the FDA on T1D and tear down its regulatory barriers. One of its major contributions to that effort was to build the first-of-its-kind registry on T1D patients. It started in 2007. “We kept on asking when we started where the data was to show how people in the U.S. are doing with T1D,” Panzirer recalled. “The data did not exist. It was mostly thought of as a safe and managed disease.” About three years later, during JDRF’s campaign to turn the FDA around (described in the next chapter), Panzirer presented the registry data to the agency. “The registry collected data on 3,000 people in 27 clinics and showed that T1D was neither managed nor safe,” said Panzirer. “I presented the data myself and their chins were on the floor.” Said Kowalski, “The registry was very helpful. It was super important to help show the unmet need.”
HCT also stepped in to beef up JDRF’s team. In 2010, with the work expanding and the possibilities for success growing, HCT provided a transformational grant providing resources for JDRF to hire an expanded team for the AP campaign, including Campbell Hutton, a regulatory expert, scientist Sanjoy Dutta, Linda Johnson, an expert in managing alliances with industry, and Marlon Pragnell, another research scientist. “It was huge,” said Soler. “They became central members of the team.” “HCT provided resources that allowed us to take the JDRF Artificial Pancreas project to the next level,” Kowalski said.
HCT would also become a leading funder of the multi-hormonal “bionic pancreas” that Boston University researcher and T1D parent Ed Damiano was developing. The Kowalski roadmap had included such a system as the final Box Six, the ultimate manifestation of a real pancreas. But JDRF’s research priorities focused in these years around systems it felt were more realistic from a developmental and business standpoint, and HCT filled the gap by investing in Damiano’s project.
In 2012, Panzirer shook things up a bit more. With concerns about CGM sensors continuing, Panzirer approached JDRF with a proposition. “I went to them and said that the sensors are not good enough and that Helmsley is going to put up $12.5 million and I want you guys to match it, and we will do an RFP to companies who can improve sensors,” Panzirer said. Kowalski remembers that initially the idea of potentially providing direct funding to companies like Medtronic did not go over well at JDRF. Kowalski said the idea that JDRF would collaborate with the largest medical device maker in the U.S.” was very controversial. “But in the end, the two combined to provide $17 million to Medtronic to improve sensor technology. Notably, the deals contained royalty rights if the grants resulted in actual commercial products. While those sensor investments did not immediately bear fruit, they would ultimately result in meaningful improvements in Medtronic sensors that are currently coming to market. “To date,” says Panzirer, “we’ve gotten $42 million in royalties back.”
The other force multiplier was NIDDK. Prior to the launch of the AP Project, NIH had for years been providing small grants to researchers dabbling in AP research. But as JDRF stepped up its campaign, NIDDK would join it, leveraging funds from the Special Diabetes Program that JDRF helped create and sustain. Four years into the project, in 2009, it disbursed $23 million in research grants that added major accelerant to the project, and continued NIH funding eventually led directly to algorithms that are being used today in several commercial AP systems.
Chapter 10: The Regulators
Five years into the project, there was cause for optimism. But the FDA remained a major problem, even an obstacle.
From the start of the AP Project in 2005, there was frustration with the position of the FDA towards the technology and its potential. By 2010, despite the approval of Kovatchev’s simulators to replace mice trials, these frustrations were boiling over as FDA was continuing to raise barriers to conducting clinical trials, and generally showing little interest in advancing the technology. In 2010, Brewer became CEO of JDRF. “The FDA had been very unhelpful. There were people there overseeing the field who didn’t think there was any need for technology to manage insulin and glucose. They felt there was already an acceptable treatment and people should do what their doctor directed, take insulin as prescribed, and they would be fine,” recalled Brewer.
JDRF and researchers were pressing FDA to allow outpatient trials not just ones in highly supervised hospital settings. And it wanted the agency to develop an Artificial Pancreas Guidance, essentially a roadmap laying out the steps that needed to be followed to get device approval: what trials are required, how they should be designed and what their endpoints should be, how many patients had to be included and what ages, what technology might be used to capture data like blood-glucose levels, among other key regulatory issues.
“There were multiple years of FDA wanting more and more data before trials could progress, which we saw as over-regulation,” recalled Kowalski. “So, frustration boiled and boiled and boiled, and we finally went to Jeff Shuren who was the new head of the Center for Devices and Radiological Health (CDRH) at the FDA and asked him to get involved.”
But they weren’t getting anywhere, so in July, JDRF brought together a broad set of stakeholders to develop recommendations on how to conduct outpatient trials safely. “In November we presented the recommendations to the FDA and other experts at an FDA/NIH public workshop,” Rice said.
It did not go well, and JDRF decided to turn up the heat.
“We were so concerned with the dismissive reaction from the lead FDA reviewer at the workshop that the same day, right after the workshop ended, we went to Larry Soler’s house nearby to confer about next steps—including me, Aaron, Jeffrey, Larry, Campbell Hutton (the in-house JDRF regulatory expert), and our regulatory consultant Phil Phillips,” recalled Cynthia Rice. “At that meeting, we decided we would draft an actual guidance document, in the format that the FDA could release, based the earlier recommendations.” This would lay out a pathway to get to the “Holy Grail” of Box Six in the Kowalski roadmap. The guidance document was submitted to the FDA in March 2011.
Over the next six months, JDRF waged an intensive, multifront advocacy campaign to send a message to FDA that it was time to move faster and more constructively on AP systems. It started in March when volunteers from all over the country flew into Washington, DC for JDRF’s annual Government Day and swept across Capitol Hill asking lawmakers to sign a letter being circulated by the House and Senate Diabetes Caucus Co-Chairs7 to the FDA to promptly issue a guidance that reflects the view of expert clinicians and researchers.
In June, JDRF staged its biennial Children’s Congress to educate lawmakers about T1D. Long-time JDRF ally Senator Susan Colins (R-ME), Chairman of the Senate Aging Committee, agreed to schedule a hearing to press FDA officials about the lack of progress in advancing artificial pancreas systems to patients. The stagecraft would be designed to create an emotional exclamation point on the issue as the 200 delegates between the ages of 5 and 17 sat in chairs and on the floor in front of the dais in the hearing room.
FDA was getting the message and tried to get ahead of things. It released a guidance document the very same day of the hearing. The bad news is the guidance was narrow—it only covered a pathway for a low glucose suspend version of the AP—Kowalski’s Box One, a first-generation device already available and in use in more than 40 countries around the world that researchers already felt they could leap beyond. Not only was it viewed as too conservative, but there was also another problem: said Rice: “It was terrible.”
In September, joined by key clinician groups like ADA, individual researchers and clinicians, JDRF submitted comments blasting the FDA’s June draft. In October 2011, it launched an online petition calling on FDA to issue a pathway to accelerate AP development. In short order, over 120,000 people signed the petition, most with a direct connection to T1D. And one month later, Senators Collins and Shaheen held a press conference, joined by an 11-year-old with T1D, Caitlin Ryan, urging the FDA to issue clear and reasonable guidance for AP that avoids unnecessary delays. A foot-high stack containing all the names of the petition signers was a prominent prop.
The final salvo came that same day. Full-page ads appeared in The New York Times and Washington Post headlined: “The FDA can help save the lives of those with type 1 diabetes.” Pictured on the top of the ad was an adorable young girl named Piper, an eight-year-old who had been diagnosed at age 3. The ad copy said: “Piper has type 1 diabetes. One in twenty people like Piper will die from low blood sugar. In fact, kids and adults are dying every day from low blood sugars or complications caused by type 1 diabetes. In the next few weeks, FDA has a chance to show it is leading the world in medical innovation not standing in its way. It will lay out the pathway to bring to market the first artificial pancreas, a lifesaving technology now under development. And the most revolutionary treatment in diabetes since the discovery of insulin. Three million kids, teens, and adults with type 1 diabetes are counting on the FDA to get it right. Our lives and health are at stake.”
To this day, people at FDA at the time, JDRF staff, and volunteers, remember “the ad.” It remains the single most public and aggressive action the organization has ever taken to advance research to treat and cure T1D.
In December, FDA released a second draft guidance on outpatient testing of AP systems. But it too didn’t meet the expectations of the T1D community. Once again, JDRF made clear to FDA that it was still falling short.
Shuren, who plans to leave the FDA by the end of 2024, remembers this period well. “JDRF asked me to meet with them and they laid out their concerns,” he recalled. “In particular, they talked about problems with the office and the FDA team. So, I started having a series of meetings with the team, JDRF, and others; and it became pretty clear to me, although they were well-intentioned, we were not engaging in a constructive manner.”
Shuren got the message, and he made his move. He decided to pull oversight of the entire AP project from the original group. “I determined that it made more sense to move the AP technology [and insulin pumps] to another part of the center that dealt with other diabetes related technology like glucose meters. So, I put all diabetes related technology in one place.”
Stayce Beck, a bioengineer, was the lead member of the new team. “The ads changed the trajectory of my life,” she said years later. “It spurred FDA to take the AP and give it to my group and they asked me to lead the project.” Early in 2012, the new team took the JDRF draft guidance “to heavily resource our version” and “to finalize it in a way that aligned with what they were thinking.” It took another 10 months but finally, in November 2012, FDA issued a new and improved guidance.
Finally, the AP community had leaders at the FDA who made clear they were there not to obstruct but to facilitate while staying true to the agency’s highest standard of ensuring any approved system was deemed safe and effective.
Said Shuren: “I do give JDRF credit for pushing, for saying there’s a real need for this, and raising the clarion bell on concerns with the center.”
“The companies would always tell us that FDA would never allow this to happen,” said Kowalski. “But now they had a guidance document from FDA saying it can happen if you do these trials this way, and the trials were the right ones, so the guidance was a seminal moment. It was huge.”
“The guidance opened the floodgates,” said Dutta. “Now companies knew how to do trials, who to recruit, how long to do them for, what do they need to prove. Now people knew there was a path as opposed to driving in the dark.”
Indeed, the research community did move into overdrive. Outpatient trials were launched and completed in waves. Kovatchev, Cobelli , Buckingham, Hovorka, a team at Mt. Sinai in New York and the Mayo Clinic in Minnesota, Ed Damiano at Boston University, all conducted trials of patients in different settings with different algorithms and system components between 2012 and 2016. They started with overnight outpatient trials, moved on to multiday in-home trials, and then to three-month and then six-month “real-life” trials. There were trials in diabetes camps and trials at ski camps. The pace was dizzying but the results were exhilarating. Time and again, the trials demonstrated both the safety and efficacy of automated insulin systems.
[10] Senators Susan Collins (R-ME) and Jeanne Shaheen (D-NH) chaired the Senate Diabetes Caucus and Representatives Diana DeGette (D-CO), and Ed Whitfield (R-KY) chaired the Congressional Diabetes Caucus.
Chapter 11: The Hackers
Progress was happening. But for many with T1D it was agonizingly slow. Most could only wait. Some decided they were tired of waiting.
Bryan Mazlish had founded an automated stock trading business where he and his team wrote algorithms to buy and sell stocks. His wife Sarah had been diagnosed with T1D when she was 12. In 2011, their middle child, Sam, was diagnosed with T1D at age 5.
“I am embarrassed to say how little I understood what my wife was going through on a daily basis until I was helping my son,” said Mazlish. “I never really understood the pernicious impact that the disease has on someone. You’re essentially driving a car on a curvy mountain road 24 hours a day, seven days a week with no time off even when you’re sleeping.”
Sam started wearing a CGM, but the couple was frustrated with the technology, largely because it was hard to hear the system’s alarm go off at night signaling a problem. “We wanted to be able to hear our son’s alarms at night and also monitor his glucose,” said Mazlish. Working with his father-in-law and brother-in-law, both engineers, they managed to figure out a way to remotely monitor Sam’s glucose levels and ensure they would hear the alarm when Sam was in trouble.
Excited about this step forward to make their son’s life safer, Mazlish asked his wife what else they could do, “and she said she had just had a rough night and if she could just wake up with a perfect blood sugar every morning that would be amazing.” The lightbulb went off.
Sarah and Bryan designed a do-it-yourself (DIY), or hacked, prototype artificial pancreas using an android phone and an algorithm they created to communicate with the pump and CGM. Sarah was the beta tester and wore the system the first night after the pair felt they had debugged it. “The first night she wore it, she slept one of the best nights of her life, but I was basically up all night monitoring the machine, making sure nothing bad happened,” said Bryan. Sarah used it for a few more months, they continued to tweak it, and when the couple were completely comfortable in it, they put it on Sam.
One other person was excited about the system. Aaron Kowalski. “Bryan calls me one day and says he wants to show me what he did. So, he comes to 26 Broadway (JDRF’s headquarters) and shows me his cell phone and it has his son’s glucose reading on the screen. I was like, ‘what the hell, what is this?’ And he says, ‘Oh, it was really easy. I just hacked it.”
Word started getting around about the system in T1D circles and Mazlish tried to get companies interested. “I talked to all the existing device makers, CGM companies, pump companies, and showed them what we had done, the data from Sam and Sarah and how amazing it was,” said Mazlish. “I said I would come work for them. But there were a lot of questions about whether FDA would approve it and a lot of hesitation and not a lot of urgency.”
Indeed, FDA was direct. “We were absolutely unenthusiastic,” said FDA’s Stayce Beck. “We did not want people to resort to these systems. We wanted to give them approved options.” If it was made available to others, even at no cost, the device would require FDA approval. In the end, the agency sent Mazlish a message: if you don’t try to promote this or make it widely available, we won’t act against you.
But by then others had taken up the DIY mantle.
Dana Lewis was diagnosed with T1D when she was a 14-year-old girl in high school in Huntsville, AL. As an adult she moved to Seattle in 2010 where she worked for a nonprofit hospital system helping how it and its doctors could effectively use social media as a communications tool. In 2013, she met Scott Leibrand, a network architect who would become her husband. Frustrated with existing diabetes management tools, they began to tinker with existing systems and in the summer of 2014, they met Ben West, another hacker who joined their work.
“By late November we had closed the loop,” said Lewis. They had in effect created a hacked AP system that predicted and prevented high and low blood sugars. In short order, the team built a website to tell the world about the OpenAPS, an open-source system that would allow others to replicate their creation. In this case, the FDA was powerless. “We weren’t distributing anything but ideas,” said Lewis. “That’s not against FDA rules and we felt pretty comfortable with open source and sharing it.” A few years later, a programmer, Pete Schwamb, took on the challenge of developing a hack that would allow users of the popular Insulet Omnipod pump to also adopt a hacked AP system.
Brewer, who would eventually leave JDRF to start Bigfoot Biomedical, with Mazlish and others believe the DIY movement played an important role in motivating the FDA to move faster. “DIY drove FDA crazy,” said Brewer. “It caused the FDA to put pressure on companies to move faster, they wanted it to go away and so they became advocates for companies to step up their game and move quickly to get away from what the FDA thought was the ‘crazy’ stuff people were doing themselves.”
Chapter 12: The Patients
Starting in 2008, hundreds of kids and adults with T1D around the world volunteered for clinical trials to test the emerging AP systems. Early on, the risks were real, and it took a lot of trust for people to rely on new-fangled technology to keep them safe. But they did it anyway.
Tom Brobson was diagnosed with T1D in July 2004 at the age of 44. “I was not the typical adolescent with T1D, and I was just trying to cope and remap my life. Then I saw Mary Tyler Moore8 being interviewed by Larry King on CNN and I donated to JDRF.” One year later, while working at Virginia Tech as a development official, he heard that JDRF was seeking to hire a major gifts officer, so he applied for the job, and was hired. Brobson would soon become one of the most visible and inspiring JDRF figures when he became one of the first persons in the world to wear a closed loop AP system as a participant in Boris Kovatchev’s hospital trial in 2008.

“They have two laptops set up,” he recalled of the experience. “I have two sensors on me. And I have an insulin pump. The two sensors were in case one failed. It was crazy just to walk down the hall to the bathroom because they had to put everything on big metal cart and wheel me down with all the wires connected. I ate dinner and watched the algorithm do a better job than I had done ten days prior. Most of all, that night, the big difference was I slept. When I ran things, I bounced low like six times through the night but when the system took over and ran things I avoided all that. And I remember sitting there texting Aaron and saying, ‘Oh my God, this thing works.’ It was a powerful moment.”
Brobson was also in the first UVA outpatient trial where the technology had transitioned to a portable system with a smart phone linked wirelessly to a glucose sensor and insulin pump. “We’re walking around with these phones with a stoplight design, a red, green, and yellow light with green indicating you were in a safe range, yellow that you were moving in the wrong direction, and red meaning that you were having a low or high blood sugar,” recalled Brobson.
Joshua Davis was diagnosed with T1D in 2009 when he was 11 months old. Joshua’s dad Brian had been diagnosed with T1D in 2004 right before his tenth high school reunion, so the family had some familiarity with the disease. But dealing with an infant was a whole different challenge.
“It was heartbreaking and just completely devastating,” said Shannon Davis, Joshua’s mom. “I went from learning how to carb count breast milk and baby food at diagnosis, because at the time that’s all he was eating, then carb count goldfish cookies when he was a toddler, figuring out how many goldfish did he throw on the floor. You see other moms pushing their babies in a stroller and they’re just kind of handing ’em food while you’ve got to stop and count each tiny goldfish that you’re giving your child. And then the sleep, we just didn’t sleep. Typically, we would check his blood sugar before he went to bed, and then we check it again at about 10 pm, then about 1 am, and then about 3 am, and then we would try to get until 7 am. Joshua actually learned how to eat a banana in his sleep to elevate his blood sugars.”
At age 5, Joshua had the opportunity to participate in a UVA outpatient trial. His parents said the choice was his. “I was really excited because I was already doing sports so the idea of being able to run around without having to worry about going low or having to stop and eat a snack in the middle of playing was a freeing feeling,” he recalled.

Joshua and Shannon headed to the Wintergreen Resort in Virginia where they would room together while Joshua wore the AP system. For several years Joshua had been on a CGM and a pump, so Shannon was used to responding to the alerts and helping her son make safe decisions on dosing insulin. But the camp was a big change.
“I can vividly remember the first night of the trial,” said Shannon. “The doctors said we needed to trust that they are watching our kids on a computer, and they told us we were not to respond the way we normally would so the system can do what it needs to. And I remember when Joshua woke up that first morning, he said he felt so good and I said, ‘Baby, you slept the whole night.’”
Alecia Wesner was diagnosed with T1D in 1979, and her parents became active with what was then called JDF. Growing up, she had two dangerous lows where she fell into unconsciousness. Eventually, she wound up in New York City in 1998. Soon, though, she was diagnosed with diabetic retinopathy, one of the common complications of T1D that can lead to blindness, especially if a person does not maintain excellent blood-sugar control and seek available treatments. As the AP Project took root, Alecia joined the local JDRF chapter. “At one of our Board meetings, Tom Brobson spoke, and he talked about being in trials at UVA, and I said I need to do that.”
She wrote to the UVA team, and they told her there was an upcoming trial that she qualified for at UVA. Months went by and then she was notified that they were no longer needed for that trial because it was no longer necessary as the technology had moved forward. But the email concluded by revealing that the next phase of AP trials were moving to other test locations including Mt. Sinai Hospital in Manhattan and she should contact the lead principal investigator at Mt. Sinai, Dr. Carol Levy. “When I got the email, I was sitting in her office.” Carol Levy was Alecia’s endocrinologist. Alecia got into the trial.

“It was amazing. I went to work every day. Nothing in my life was different. And then after work I would rush back to the hotel and they would set everything up and once we were in bed for the night, we got to turn the unit on. And they observed us while we slept. I knew from my own experience that I had been having problems going low at night then bouncing back up in the morning and sleeping through the alarms from my CGM. With this AP system, every day I woke up with glucose levels in range, so however my day started I was not chasing a high or low blood sugar.”
Looking back at her motives for volunteering, Wesner said: “Getting involved in AP trials to me was my chance to pay it forward for somebody else. I have lived 45 years with T1D, and I have lost friends to type one. And I think there’s an enormous responsibility that comes with being alive. I think there’s something comforting in knowing that my body was used for something that not only had the potential to make me healthier, but really was for other people. I do think there’s something to be said for doing good, feeling good, and this is what it felt like being part of trials.”
[8] Mary Tyler Moore had been diagnosed with T1D around 1970 at the age of 33. She soon became JDRF’s International Chairperson using her fame to raise awareness of the disease, increase private and federal research support, and testifying before Congress and inspiring others with the disease to live their lives fully and boldly
Chapter 13: The Finish Line
Spurred by the FDA guidance, goaded by the JDRF Animas deal9, the 800-pound gorilla finally come down from its mountain habitat and got into the game. Fittingly, it was FDA that played a key role in catalyzing the company’s involvement. “FDA would tell us flat out they were begging Medtronic, which was amazing, it was a 180-degree turn, like going from incredible headwinds at to having the wind at your back,” said Kowalski.
“In 2013 we reached out to Medtronic because they had a pump, a CGM, and an algorithm and we said we want to make this happen,” said Stayce Beck. “Let’s figure out what we need to do. So, we ended up meeting with Medtronic monthly to get them to accelerate. They originally weren’t planning on being in the market until 2019.”
In mid-2016, Medtronic announced that it had completed a trial of a hybrid closed loop system called the MiniMed 670G. The trial included 123 adolescents and adults at nine sites in the US and one site in Israel. It was a so-called pivotal trial, the last step in the drug and device development process before applying to the FDA for approval. Participants on the system for three months experienced a 44% reduction in time spent with a low blood sugar, a 40% reduction in dangerous low-blood-sugar territory, and an 11% decline with dangerous high blood sugars.
Just three months later, acting with unprecedented speed, on September 28, 2016, the FDA approved the 670G, and the Artificial pancreas Project had achieved its goal.
[9] In 2017, Animas exited the diabetes market without ever completing work on an AP. Speculation was that the company did not move quickly enough and was eclipsed by new iterations of the original Medtronic 670G.
Chapter 14: The Aftermath
Since the approval of the 670G, FDA has approved four additional systems, including the T:Slim X2 by Tandem, using the original algorithm developed by Boris Kovatchev, the Omnipod 5 by Insulet, using the algorithm developed years earlier by Frank Doyle’s team at UCSB, and Tandem’s Mobi, the Cam APS developed by Dr. Roman Hovorka’s team at the University of Cambridge in England, the iLet Bionic Pancreas that emerged from Ed Damiano’s team at BU10, and most recently, the Dean Kamen designed twiist from Sequel Med Tech. Thousands of others continue to use DIY systems.
As readers will recall, the landmark Diabetes Control and Complications Trial, or DCCT had found that people with T1D could reduce the risk of long-term complications by 35-76% through extremely tight blood-sugar control—keeping their levels in a range that largely eliminated dangerous highs and lows. But, as Brewer discovered when his son was diagnosed, meeting these ideal targets was nearly impossible with the technology in use in 2005.
Today, two decades after Brewer and Kowalski persuaded a skeptical JDRF to launch the AP Project, the latest available data leaves no doubt that their belief that developing AP systems would improve lives and reduce the risk of long-term health complications for people with T1D has been vindicated.
In June 2024, the T1D Exchange, a nonprofit dedicated to improving outcomes for people with T1D, completed a study11 of people living with T1D and found that children under 13, teens, and young adults between ages 18 and 29 using hybrid closed loop systems were achieving safe target blood-sugar levels 40-68% more often than those using insulin pumps and CGMs. Results were slightly lower for adults over 30 years old but still were statistically significant. The T1D Exchange data also showed that young people tended to have fewer severe low blood-sugar events on hybrid closed loops than those on pumps and CGMs.
“What we brought to bear is resulting in a safer and easier life for hundreds of thousands, and soon millions, of people with T1D, including my son, that is going to keep them safe until something like a cure comes along,” said Brewer.
[10] Work still continues on the elusive Goal Six multi-hormonal system.
[11] Automated Insulin delivery Use among 12,065 T1D Exchange Registry participants
Postscript
Pregnant women with T1D have a host of challenges and risks. They are eating for two, they have varying levels of stress, they have additional hormones impacting them systemically, including hormones that can upset blood-sugar control. In fact, one of two babies born to women with T1D have complications, most commonly preterm birth, large birth weight, and admission to neonatal ICUs. In 2008, Dr. Helen Murphy, a UK clinician who works with pregnant women with T1D, started collaborating with Roman Hovorka at the University of Cambridge, one of the early artificial pancreas research pioneers, who developed the CamAPS (artificial pancreas system) which was approved in the U.S. by FDA in May 2024.
Dr. Murphy’s focus was on using the AP systems to improve pregnancy outcomes for T1D Moms. Early systems were not sufficiently reliable to risk using with pregnant women, but by 2023 the time was ripe. Hovorka, Murphy, and colleagues around the UK conducted a trial of 124 pregnant women with T1D to test whether the Cambridge AP system could improve maternal glucose enough to benefit pregnancy outcomes. All users of this system experienced 10.5% more time in range throughout their pregnancies. Soon after, the UK’s National Institute of Health Care and Excellence issued clear guidance advising that all pregnant women in the UK be given access to pregnancy-specific AP systems. More than forty years after Bill Tamborlane asked Dean Kamen to develop a pump for his work with pregnant woman, the technology had become standard of care.
In February 2024, my daughter, diagnosed with T1D at age 14 in 2001, gave birth to her second child. She had her first in 2020. She wore the original DIY version of the Omnipod closed loop system in both pregnancies. Both babies were born healthy and on time.
“The loop system has truly transformed my life, making everyday activities easier and less stressful,” Emma said. “It allowed me to experience both of my “high-risk” pregnancies as “normal,” with tighter control over my blood-sugar levels so I could focus on enjoying the prenatal and postpartum journey. Day to day, this technology has given me greater stability and peace of mind, letting me live life fully without the constant worry of managing my diabetes.”
At the 2024 International Society for Pediatric and Adolescent Diabetes (ISPAD) Conference in Lisbon, Portugal, the world’s leading diabetes researchers, academics, and members of industry gathered to share the latest and greatest in diabetes research. Breakthrough T1D leaders and many of our funded researchers and collaborators were on hand —for ISPAD’s 50th birthday—to share new science, breakthroughs, and a glimpse at what the next generation of T1D therapies will look like.
Let’s look at a few highlights!
The T1D Index Levels Up

Breakthrough T1D Chief Scientific Officer Sanjoy Dutta, Ph.D., and Thomas Danne, MD, Chief Medical Officer, International presented a much-awaited update on the T1D Index at ISPAD.
The T1D Index is a first-of-its-kind data simulation tool that measures the human and public health impact of the T1D crisis in every country across the globe. Until the creation of the Index, there have been wide gaps in the data about the incidence and impact of T1D. Leveraging data and insights from the T1D Index can help change the lives of people living with T1D by identifying attainable country-by-country interventions, including timely diagnosis, accessible care, and funding research that could lead to cures.
First , Dr. Dutta gave a refresher on our recent brand evolution, which includes why this change was necessary and how our research strategy has not changed.
Dr. Danne followed with a reintroduction of the Index, its place in our mission, and how we improved it. This includes:
- An enhanced dashboard and user interface designed to improve user experience
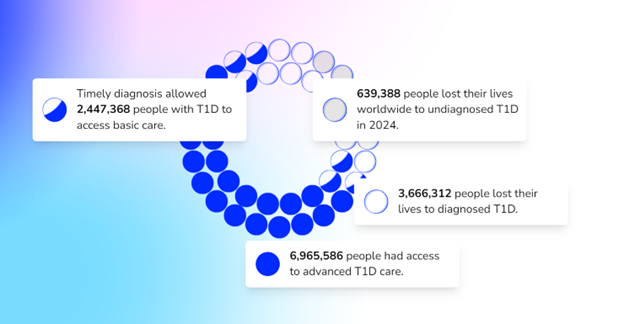
- A plan to develop Index 2.0
- An invitation and plea for all countries and existing registries to collaborate with data to enhance and develop Index 2.0
- Advanced simulation capabilities allowing for scenario analysis.
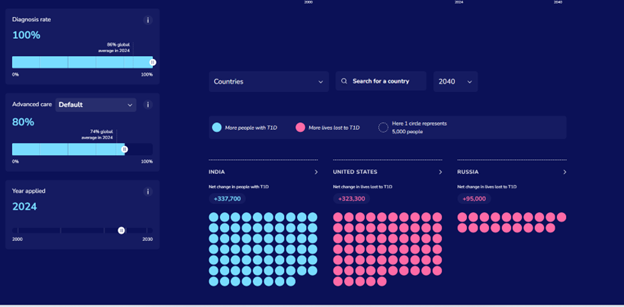
- Validation of data against available national registry data.
- Performance upgrades, including improved platform speed and minor adjustments to further refine accuracy.
In the future, the Index aims to have even more detailed data on a regional level.
Key takeaway:
Breakthrough T1D is focused on improving the lives of people with T1D all around the world—that includes every country in the world. The Index is key to defining the global scope of T1D and the country-by-country solutions to bring better outcomes to everyone affected by T1D.
Is metabolic control enough for young people with T1D?
We have known for decades that T1D is accompanied by the risk of developing complications. The conditions that are most associated with T1D include diabetic eye disease (retinopathy), kidney disease (nephropathy), and heart disease (cardiovascular disease). This is not an exhaustive list—there are many others, including mental health disorders. These complications most commonly occur in adults living with T1D, but new research suggests there’s more we can do to monitor and intervene with these complications in youths with T1D in addition to striving for ideal glycemic control.
At ISPAD, Breakthrough T1D-funded researcher Risa Wolf, MD, discussed long-term microvascular and macrovascular complications in T1D, including the complications mentioned above, and the data is striking. There is a low percentage of complications 4-7 years after diagnosis but after 12 years:
- 24% will have a decline in eGFR (a biomarker for kidney function)
- 13% will have neuropathy
- 52% will have retinopathy
- 28% will have dyslipidemia (abnormal levels of lipids in the blood)
Thankfully, leaps in care management, including automated insulin delivery systems and continuous glucose monitors, have led to improvements in A1c and, in turn, the rate at which people develop complications. But more can be done.
Through regular screening for these more common complications for all youths with T1D, we can better identify, diagnose, and monitor individuals as they develop these complications—especially because many of these screenings are low-cost.
Breakthrough T1D, for example, is funding Dr. Wolf to use the autonomous AI system (IDx-DR, Digital Diagnostics), which is FDA-approved for adults 21+ to detect and diagnose diabetic retinopathy in children. Ideally, this will lead to FDA approval and a better tool for monitoring this complication in kids.
Drs. M. Loredana Marcovecchio – another Breakthrough T1D funded investigator and Didac Mauricio took a similar look at vascular complications. Their presentations had related themes—it is critical to develop ways to identify complications like cardiovascular disease earlier. If they’re identified earlier, individuals can take advantage of the approved therapies.
Key Takeaway:
Like most areas of T1D management: there are still significant unmet needs.
We need to have a long view of these complications and their pathogenesis, which can start in youth. Glycemic control is critical to preventing the onset of these complications, but it is not always enough. Cardiovascular disease, retinopathy, dyslipidemia, and more can all be detected at a young age, which gives more time for interventions.
Breakthrough T1D is working on all of this in our Improving Lives program.
ISPAD / Breakthrough T1D Sessions
Breakthrough T1D Chief Scientific Officer Sanjoy Dutta, Ph.D. joined several collaborators for two sessions.
In the first, titled “Joint-Symposium Breakthrough T1D (formerly JDRF)-ISPAD: Type 1 Diabetes (T1D) Treatments: Hope and Promise Ahead,” two recipients of ISPAD/Breakthrough T1D fellowships, which allow care providers to travel to and receive training at world-class diabetes centers, shared their experiences and how the fellowship enabled them to improve the care they can provide to their communities.
After a presentation from Chantal Matthieu, a longtime Breakthrough T1D investigator, partner and reviewer, Dr. Dutta presented an overview of our work, our vision, and how the next generation of researchers in the room can apply for funding and join us in our work.
In the second session, the Joint ISPAD – Breakthrough T1D symposium, Dr. Dutta and others provided an update on our progress in treating this disease and what’s on the horizon. They touched on the many ways we can slow down disease progression, the exciting state of stem cell-derived cell therapies (they’re in human clinical trials and they’re producing insulin), and how artificial intelligence can play a role in reducing or eliminating T1D misdiagnosis in adults which can be as high as 40%.
Key takeaway:
It’s an exciting time in T1D research, and Breakthrough T1D’s strategy has mapped out what it takes to get to cures—including the importance of bringing in the next generation of researchers to contribute.
Revolutionizing Diabetes Care: Cutting-Edge Therapies
This session, which was a personal favorite of the author, gave updates on the biggest priorities we have at Breakthrough T1D: cures and how close we are to realizing them.
Spoiler alert: we’re not that far away.
It featured presentations from several Breakthrough T1D-funded researchers, including Colin Dayan, MD, Lori Laffel, MD, and Kimberly Simmons, MD.
First up: Dr. Simmons spoke about Tzield, the first disease-modifying therapy approved for T1D, and its use in the real world. A quick summary of the data:
- 322 people have received the drug to date
- The side effects reported in the clinical trial are consistent with what has been seen at the Barbara Davis center
- “Most of our patients have told us that they would do this treatment again.”
- Long term data for how people are tolerating the therapy and progression to T1D is not available yet.
As a reminder, Tzield was FDA-approved in November 2022 for individuals with stage 2 T1D to delay onset by an average of two years. Learn more in this comprehensive story on how it came to be and the role Breakthrough T1D played in getting it to market.
Dr. Michael Rickels then explored cell replacement therapies: where we are and where we’re going. His presentation covered the current landscape: We can use cadaveric islets to restore insulin production in people with T1D. However, the requirement for chronic immunosuppression and scarcity of donor islets mean this is not a viable option for the vast majority of people with T1D.
The next step is using an unlimited supply of stem cell-derived islets and discovering methods to keep them safe without the use of chronic immunosuppression, which is consistent with our strategy in this area.
Dr. Rickels also touched on the study that used autologous cells to restore insulin production in a person with T1D.
Lastly, Dr. Colin Dayan gave an update on combination therapies to preserve beta cell function in people with T1D.
Combination therapies utilize several different therapies that work by discrete mechanisms to target a singular problem. An example is tuberculosis or HIV, which both relied on complicated treatments composed of different pills that had to be taken at different times of day but are now treated with singular pills.
As we have shown, there are many drugs that can preserve insulin production. Now we’re learning which drugs can be taken together to preserve that insulin production longer. Dr. Dayan discussed the T1D Plus study (which builds on the Ver-A-T1D trial), which is funded by Breakthrough T1D, JDRF Australia, INNODIA, and the Helmsley Charitable Trust. This study will test several different drug combinations to see their effectiveness, including Tzield, verapamil, antithymocyte globulin (ATG), and golimumab.
Not only will this trial test multiple drugs—the innovative design of the study will speed up the length of the study, shaving years of the time it will take to see what combinations work best.
Key takeaway:
Breakthrough T1D is at the center of cell therapies and disease-modifying therapies. The clinical trials being run in humans today are very exciting and are a glimpse into how this disease could be treated in the not-too-distant future. It’s possible there will be approved combination therapies to slow progression and cell replacement therapies that restore insulin production entirely in the not-too-distant future.
Until next year!
These are just a few highlights from three days of sessions and presentations that cover our entire research portfolio. For example, Dr. Danne presented on the importance of early detection due to its numerous, clearly defined benefits and strategies for identifying these youths with stage 1 and stage 2 T1D. There were also sessions on the impact of AID systems, specific areas of complications, and much, much more.
We’re already looking forward to next year!
The American Diabetes Association’s 84th Scientific Sessions is here! Scientists will present the latest type 1 diabetes (T1D) research, from early detection to glucose control to complications, all with the goal of improving lives for the T1D community.
- In November 2022, the FDA approved Tzield™ (teplizumab-mzwv) for use in delaying the onset of clinical T1D. With the availability of a treatment option for people with Stage 2 T1D, the field has changed its outlook on delay and prevention and navigating pediatric T1D, especially in the early stages. Annette-Gabriele Ziegler, M.D., presented on several screening programs in Europe, including Fr1da, which has screened 200,000+ pediatric participants and found that it significantly reduces DKA onset at clinical diagnosis, and GPPAD, which identifies infants with an elevated genetic risk of developing T1D and enrolls them in primary prevention clinical trials. Andrea Steck, M.D., highlighted the value of CGM-based metrics in evaluating T1D risk and R. Brett McQueen, Ph.D., discussed the economics of early detection.
At-risk, or Stage 2 T1D, means that a person exhibited 2+ T1D-related autoantibodies—antibodies against one’s own self—and their blood glucose is starting to be abnormal, but they are not yet insulin dependent. When someone becomes insulin-dependent, they are in stage 3 T1D.
- We got updates on several automated insulin delivery (AID), or artificial pancreas, systems, including the:
- Medtronic MiniMed 780G, especially the importance of initiating it as soon as possible following diagnosis (which is now recommended in the ADA Standards of Care for both children and adults), citing the CLVer trial, which found clinically meaningful and sustained improvements in blood sugar management following early AID initiation.
- Medtronic MiniMed 780G in high-risk youth with T1D, with 80 participants aged 7-25 years, who demonstrated an average HbA1c reduction of 2.5% (from an average baseline HbA1c of 10.5% to 8%), improvement in time-in-range, and a reduction in low blood sugar events.
- Tandem Mobi among early pediatric and adult adopters.
- Sequel Med Tech’s twist AID system, which was FDA-cleared for people with T1D aged 2+ in March 2024. The system uses the DEKA Loop algorithm, which is based on the FDA cleared Tidepool Loop (iAGC) and is intended for use with compatible interoperable continuous glucose monitors (iCGMs). Sequel is Tidepool’s first publicly announced insulin delivery device partner with an FDA-cleared system that will integrate Tidepool Loop.
- In therapy, the full INHALE-3 results demonstrated the non-inferiority of inhaled insulin (Afrezza) used with insulin degludec compared to usual care. Baseline HbA1c was 7.6% across groups, and, on average, HbA1c in both groups remained stable from baseline to 17 weeks. Overall, 30% of the inhaled insulin group reached <7.0% A1c at 17 weeks compared to only 17% of the usual care group.
As the leading global type 1 diabetes (T1D) research and advocacy organization, Breakthrough T1D helps make everyday life with the condition better while driving toward cures. We won’t stop until the condition is a thing of the past.
That means powering research to cure, prevent, and better treat T1D and its complications and ensuring that the entire T1D community has access to the tools they need to thrive.
Breakthroughs past, present, and future
For more than 50 years, Breakthrough T1D has played a pivotal role in nearly every major T1D breakthrough—from how HbA1c came to be more than 40 years ago to recent advancements like advanced T1D management devices, such as artificial pancreas (AP)/automated insulin delivery (AID) systems. Our work has also ensured that people have access to these advances.
Let’s have a look at some of the biggest breakthroughs we’ve advanced that are improving lives right now and those that promise to improve lives in the future.
The first disease-modifying therapy for T1D
In 2022, the FDA approved Tzield™ (teplizumab-mzwv) for use in delaying the onset of clinical T1D. This was the first disease-modifying therapy (DMT) for T1D to be approved. These are treatments that can slow, halt, or reverse the course of a condition. It took decades of Breakthrough T1D research for Tzield to reach approval.
Beginning with basic research by Kevan Herold, M.D., in the 1980s to preclinical and early clinical trials to a strategic investment from the T1D Fund: A Breakthrough T1D Venture that brought Provention Bio—the company that launched Tzield—into T1D for the first time, in 2017.

“The story with the clinical use of teplizumab began with a Breakthrough T1D grant to support a trial in patients with new-onset type 1 diabetes more than two decades ago. The success of this initial study planted a seed that led to further studies and support from the NIH.”
Kevan Herold, M.D.
Yale School of Medicine
But there’s a personal story, too.
Andy Drechsler and his wife Moira are the parents of four children; three of them have T1D. They have been involved with Breakthrough T1D since their son was diagnosed at the age of 22 months in 2005.
Andy’s professional life intersected with his personal life when he helped start Provention Bio in 2017.
“Everyone living with T1D provides us with great inspiration. We also appreciate the parents and caregivers of T1Ds. We are so happy to see the improvements in pumps and CGMs for those living with T1D. We are also thrilled to see therapies to delay the onset. Ultimately, we are confident that therapies will allow many to live without T1D someday.”
Andy Drechsler
Board of Directors President
Breakthrough T1D New Jersey Metro and Rockland County Chapter

We are moving ever closer to a world without this disease. Tzield is one gigantic step along the way, and others are right behind it. Read about the other disease-modifying therapies in our pipeline to learn about the drugs that could become the “next Tzield.”
T1D management devices—decades in the making
For 20+ years, Breakthrough T1D spearheaded efforts to develop artificial pancreas (AP) systems—also called automated insulin delivery (AID) systems. We would go on to fund more than 150 grants, including 50+ clinical trials, funded by us and backed by our Artificial Pancreas Consortium, to make the artificial pancreas system a reality.
Thanks to Breakthrough T1D research and advocacy efforts, approximately 15 FDA-approved T1D management devices are on the market today—more than half of them AP systems and the remainder, advanced CGMs and insulin pumps.
Read about the different AID systems that the U.S. Food and Druge Administration has approved in recent years.

“Witnessing the progression of T1D breakthroughs over the years has been nothing short of remarkable. When I first started insulin injections, it was a cumbersome routine, requiring multiple injections a day. Today, thanks to advancements in insulin pump technology, managing T1D has become more streamlined and efficient, improving my quality of life.”
Princess Padmaja Kumari Parmar
Breakthrough T1D Global Ambassador
Early detection empowers families, helps advance research
T1D develops in stages over time. Early detection identifies people who have early-stage T1D, but no symptoms, by a simple blood test. It looks for markers in their blood called autoantibodies. These autoantibodies signal that the body’s immune system is attacking the insulin-producing cells in the pancreas.
Autoantibodies also have value in identifying individuals who would later develop T1D, providing a new staging for presymptomatic T1D. The presence of two or more means that your lifetime risk of getting T1D is nearly 100 percent.
Early detection gives families time to plan and prepare before the onset of the condition, prevents life-threatening complications and hospitalization at the onset of symptoms. Critically, it also identifies at-risk people who can take advantage of preventive therapies—including disease-modifying therapies such as Tzield—or participate in clinical trials for T1D therapies being developed.
NFL Super Bowl Champion Orlando Brown, Jr., knows all too well how dramatically T1D can impact families, as his late father and his younger brother were both diagnosed with T1D.
In his role as Breakthrough T1D ambassador, the Cincinnati Bengals offensive tackle strives to educate people about T1D and the importance of T1D early detection and research. He also uses his platform to advocate for insulin affordability and policies like the Special Diabetes Program (SDP). Last summer, he was one of 10 Celebrity Role Models at Breakthrough T1D Children’s Congress.
Learn about our program, Breakthrough T1D Early Detection.
“The sudden loss of my father to diabetic ketoacidosis and my younger brother’s type 1 diabetes diagnosis at just 11 years old brought us face-to-face with uncertainty and the stigmas surrounding this condition. However, as we learned about diabetes devices and treatments to help manage the disease, we discovered a renewed sense of peace and hope. With more research, we believe we can ultimately end this disease. Knowledge is power and I’m sharing my family’s story to educate and inspire others who are living with type 1 diabetes.”
Orlando Brown, Jr.
NFL Super Bowl Champion
Breakthrough T1D Ambassador

Breakthrough in progress: stem cell-derived replacement therapies
Cell therapies replace beta cells in the bodies of people with T1D so that they can again produce their own insulin.
Biotech powerhouse Vertex Pharmaceuticals is making major headway in its goal of developing stem cell-derived replacement therapies for T1D. This work being advanced by Vertex has been supported by Breakthrough T1D for decades.
Vertex launched its clinical trial of VX-880, a stem cell-derived islet therapy in T1D for individuals with hypoglycemia unawareness, in combination with immunosuppressive therapy to protect the cells from rejection. Several people who have received VX-880 have been able to stop taking insulin.
This work was pioneered by Doug Melton, Ph.D., whose years of Breakthrough T1D-funded research led to successfully transforming stem cells into beta cells in 2014. A catalytic investment from the T1D Fund in Semma Therapeutics—the biotech company Melton founded to develop a stem cell-derived islet therapy for T1D—was followed years later by Vertex acquiring Semma. Vertex also acquired ViaCyte, which like Semma, had received support from Breakthrough T1D and the T1D Fund for its cell therapies research.
See the timeline of Breakthrough T1D’s support of stem cell-derived islet replacement therapies.

“My lab research has been for more than a decade or two, trying to cure type 1 diabetes. That might sound like an overly ambitious project, but I believe it’s a solvable problem. Our lab worked for years to figure out how to turn stem cells into functional beta cells. We can now make billions of functional beta cells.”
Doug Melton, Ph.D.
Vertex Pharmaceuticals Research Scholar
We are Breakthrough T1D
Breakthrough T1D is knocking on the door of something big. Giant leaps are happening nearly every day. You have gotten us to where we are today—and you can help us get to the finish line faster. So that you, your loved ones, and people everywhere can enjoy a world free from the burden of T1D. A world where people don’t have to manage their diabetes—don’t take insulin, don’t have blood sugar highs and lows, and don’t develop complications. With your ongoing support, we won’t stop until this condition is a thing of the past.
Learn More About Our New Brand.
Learn More About Our Organization.
In just the past year, we have had multiple artificial pancreas systems authorized by the Food and Drug Administration (FDA)…and it’s not stopping! Last week, Tandem Mobi—a miniature-sized insulin pump, for use with Tandem’s Control-IQ™ technology and a compatible continuous glucose monitor (CGM)—received FDA clearance.
The Tandem Mobi is half the size of the company’s t:slim X2™ insulin pump, making it the world’s smallest artificial pancreas system. It is fully controllable from a mobile app through a user’s compatible iPhone and is approved for people with diabetes aged 6 years or older
In 2019, Tandem was the second to gain FDA clearance for its Control-IQ™ advanced hybrid closed loop technology, an algorithm that can be used with Tandem’s t:slim X2™ insulin pump and a compatible CGM. It was the first algorithm authorized as an interoperable automated glycemic controller, which means the algorithm could be a component of any open protocol, or interoperable, artificial pancreas system.
From Tandem’s website: A limited release of the Tandem Mobi system is expected to start in late 2023, with full commercial availability expected in early 2024. Current t:slim X2 insulin pump customers, with at least 12 months remaining on their pump warranty, can find more information on how to switch to the new Tandem Mobi through the Tandem Choice Program.
Breakthrough T1D Vision and Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development, and manufacturers embraced this to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project and Consortium almost 20 years ago. Our goal: (1) To ensure that people with T1D would have better, more innovative ways to manage their disease, and (2) to enable a competitive ecosystem that drove continuous innovation.
- Through the project, Breakthrough T1D has dramatically accelerated progress by bringing together academic researchers, government agencies, industry partners, and the Helmsley Charitable Trust to pursue artificial pancreas technology.
Breakthrough T1D has funded over $140 million to date in artificial pancreas research.
- Five algorithm teams of the Breakthrough T1D Artificial Pancreas Consortium and Breakthrough T1D-funded grants made possible the algorithms in the Medtronic MiniMed 670G, 770G, and 780G, Tandem Control-IQ™, CamAPS® FX, Insulet Omnipod 5, Tidepool Loop, and the iLet® Insulin-Only Bionic Pancreas System.
- The Special Diabetes Program, which funds T1D research through the National Institutes of Health, funded the clinical trial that led to Control-IQ’s FDA clearance.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management until cures are found.
JDRF’s vision is a world without type 1 diabetes (T1D) and in the past fiscal year, through many top type 1 diabetes advances, we’ve made incredible progress toward that goal.
Your support of our efforts is inseparable from the top type 1 diabetes advances we’ve seen in accelerating cures, improving lives, and advocacy wins for people with T1D and their loved ones.
As we approach the end of fiscal year 2023 (FY23), let’s highlight the many top type 1 diabetes advances we’ve seen.
Top Type 1 Diabetes Advance 1: First T1D Disease-Modifying Therapy
In a historic moment for T1D—and one that Breakthrough T1D had a hand in from the beginning, supporting research from the 1980s on—the U.S. Food and Drug Administration (FDA) approved Tzield™ (teplizumab-mzwv) for use in delaying the onset of clinical disease in at-risk individuals aged 8+.
For the first time in history, Tzield will treat the autoimmune process behind T1D, not the symptoms, altering the course of the disease.
Among our top type 1 diabetes advances, this is the first disease-modifying therapy—treatments that can slow, halt, or reverse the course of the disease—for T1D to be approved, but it won’t be the last.
Additionally, months after Tzield’s FDA approval, Sanofi acquired Provention Bio, the manufacturer of Tzield.
The acquisition brings the first T1D disease-modifying therapy available in the U.S. into the portfolio of a global leading pharmaceutical company, representing an endorsement of the potential of these types of therapies and, we hope, the opportunity to bring this life-changing therapy and others in the pipeline to more people faster.
Tzield and breakthroughs like it put us on the pathway to finding cures and, one day, preventing T1D entirely.
Top Type 1 Diabetes Advance 2: A Blood Pressure Drug Preserves Beta Cell Function
A Breakthrough T1D-funded study found that children and teens newly diagnosed with T1D who took verapamil—a drug already approved to treat high blood pressure—were making more insulin one year after diagnosis than those on placebo. In other words, in the children and teens who took verapamil, more beta cells were healthier one year post T1D diagnosis than those in the children and teens who took the placebo.
This was the second trial that found the drug can preserve beta cells in the newly onset period.
Additional studies may be needed to further validate the results, as well as identify all benefits and potential side effects of the drug. Breakthrough T1D has the strategy to answer these and other questions.
The finding brings us closer to our goal of having numerous disease-modifying therapies widely available for people with type 1 diabetes.
Top Type 1 Diabetes Advance 3: Affordable Insulins for Everyone
Breakthrough T1D and partnering organizations are supporting nonprofit pharmaceutical manufacturer Civica Rx to produce biosimilar insulin that will cost no more than $30 a vial/$55 a box of five pens, regardless of insurance status.
One year after the Civica announcement, Eli Lilly, Novo Nordisk, and Sanofi all announced reductions to the prices of their insulins—including the most used insulins, such as Humalog, NovoLog, and Lantus.
Another big win for insulin affordability was the $35 monthly out-of-pocket co-pay cap for those on Medicare included in the Inflation Reduction Act that Breakthrough T1D fought hard to secure.
In April, the Senate Diabetes Caucus Co-Chairs, Jeanne Shaheen (D-NH) and Susan Collins (R-ME), introduced the INSULIN Act of 2023, another key step toward achieving affordable insulin for all who need it.
The bill seeks to limit out-of-pocket insulin costs by ensuring that people with commercial insurance pay no more than $35 or 25 percent of the net price per month for at least one insulin of each type and dosage form, and includes other important provisions to help make insulin more affordable and accessible.
You can contact your members of Congress and encourage them to support the INSULIN Act of 2023.
Top Type 1 Diabetes Advance 4: Turbo Boosting Cell Therapies
Breakthrough T1D is working to develop and deliver life-changing therapies that place healthy, insulin-producing beta cells back into the bodies of people with T1D. There was a lot of progress in FY23.
Vertex, which previously acquired Semma Therapeutics, also acquired ViaCyte, bringing together the leading companies developing stem cell-based therapies for diabetes.
Vertex is advancing a stem cell-derived islet replacement therapy for T1D. It’s in human clinical trials and showing amazing results, with one participant being off insulin entirely.
Vertex also started a trial with a new product using encapsulated stem cell-derived islets as replacement therapy, and is exploring gene-edited stem cell-based therapies—both with the goal of eliminating the need for immunosuppressive drugs.
Just this past April, Aspect Biosystems—an industry leader in 3D bioprinting technology—and Novo Nordisk announced a partnership to expand the development of a new class of treatments for diabetes and obesity, using Aspect’s bioprinting technology and Novo Nordisk’s expertise in stem cell and cell therapy development.
The Aspect-Novo Nordisk partnership’s initial focus will be on developing bioprinted therapies for transplant that would be designed to maintain normal blood-sugar levels without the need for immunosuppression. This could represent a transformative treatment for people living with T1D.
Additionally, the U.S. Food and Drug Administration (FDA) approved CellTrans’s Lantidra™, the first cell therapy to be authorized in the United States, for use in adults unable to approach average blood glucose levels due to current, repeated episodes of severe low blood sugar. This therapy, which requires the use of immunosuppressive drugs, takes deceased donor islets and places them into people with T1D suffering from repeated severe low blood-sugar, called hypoglycemia, events. This is an exciting first.
Approved! Numerous T1D Management Technologies
Breakthrough T1D funds research to facilitate the development of new therapies and technologies to make day-to-day life with T1D easier, safer, and healthier. In the past year, we saw:
Newly-Approved Artificial Pancreas (AP) Systems and Algorithms
- iLet® Insulin-Only Bionic Pancreas System for ages 6+
- Medtronic MiniMed™ 780G AP system for ages 7+
- Tidepool Loop, an algorithm that will allow for interoperability of continuous glucose monitors (CGMs) and insulin pumps
Newly-Approved Continuous Glucose Monitoring (CGM) Systems
- Dexcom G7® CGM system for ages 2+
A New Tool to Accurately Diagnose Type 1 in Adults
Misdiagnosing adults with T1D as having T2D is an all-too-common problem that can have tragic consequences. Breakthrough T1D and IQVIA teamed up to develop an algorithm using artificial intelligence to examine medical records and identify individuals who were diagnosed with T2D but actually have T1D. This could be used in real time to correct misdiagnoses, offering the potential for future development into a clinical decision support tool.
A First-of-its-Kind Lifesaving Tool: The T1D Index
Breakthrough T1D and other T1D-related organizations launched the T1D Index, a first-of-its-kind data simulation tool that offers the most accurate estimate of T1D ever created. The Index measures and maps how many people live with this condition in every country, the healthy years of life it takes from people living with T1D, the number of people who would still be alive today if they hadn’t died prematurely from T1D complications, and our global strategy to reduce the impact of T1D.
Go Forward
Your partnership has been crucial to these advances and many more. On behalf of our community, thank you for moving us forward and ever closer to a world without T1D.
We are excited for the top type 1 diabetes advances that fiscal year 2024 (FY24) will bring!
Read Past Blogs about Top Type 1 Diabetes Advances:
What We Can Be Proud of in 2022
What We Can Be Proud Of in 2020
Top 10 T1D Breakthroughs of 2019
The U.S. Food and Drug Administration (FDA) cleared the iLet® Insulin-Only Bionic Pancreas System, which is designed to autonomously determine and deliver insulin doses to control blood-sugar levels, for people 6 years of age and older with type 1 diabetes (T1D). It includes an algorithm and an integrated infusion pump, which communicates directly with a compatible FDA-cleared integrated continuous glucose monitor (iCGM), enabling it to be an artificial pancreas, or automated insulin dosing (AID), system.
What’s new about this system? Ease-of-use. The iLet system is designed to have users enter only their weight for the iLet to initialize therapy. Immediately thereafter, the iLet begins controlling blood-sugar levels automatically, without requiring the user to count carbohydrates, set insulin delivery rates, or deliver additional insulin for meals or corrections. (Users do have to say whether the amount of carbs in a meal is small, medium, or large, but the algorithm learns over time in response to their individual insulin needs.)
The submission was based on a multi-center randomized insulin-only iLet Bionic Pancreas pivotal trial, which tested the insulin-only configuration in 440 adults and children 6 years and older with T1D. The trial met all key endpoints, demonstrating improved outcomes over standard of care for people living with T1D:
- Average HbA1c fell from 7.9% to 7.3% at 13 weeks
- An average of 2.6 hours more time-in-range (70-180 mg/dL) per day, improving from 51% to 65% at 13 weeks
- No increased risk of hypoglycemia
There are now multiple artificial pancreas systems on the market: The Medtronic 670G (2016), Tandem Control-IQ™ (2019), Medtronic 770G (2020), Insulet Omnipod 5 (2022), Medtronic 780G (2023), and, now, the iLet® Insulin-Only Bionic Pancreas System. (Tidepool Loop, an app that contains an algorithm that automates insulin dosing, has also been approved, but it has not yet announced its insulin pump manufacturer.)
Breakthrough T1D Impact
Breakthrough T1D started the Artificial Pancreas Project over 15 years ago to ensure people with T1D have better, more innovative ways to manage their type 1 diabetes until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation. To date, Breakthrough T1D has funded more than $140 million in artificial pancreas research.
Through these grants, Breakthrough T1D supported the development of the algorithm and preclinical and early clinical research—in partnership with the Helmsley Charitable Trust—through grants to:
- Firas El-Khatib, Ph.D., who received a Breakthrough T1D postdoctoral fellowship from 2006-2007 and is now co-founder and VP, Research & Innovation, at Beta Bionics
- Ed Damiano, Ph.D., co-founder and executive chair of Beta Bionics, from 2009-2011
- Steven J. Russell, M.D., Ph.D., who received a grant from 2013-2016 and is the principal investigator on all of the iLet clinical trials
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
The Food and Drug Administration (FDA) recently approved the Medtronic MiniMed™ 780G artificial pancreas system for use in individuals 7 years and over. It provides automatic adjustments and corrections to blood-sugar levels every 5-minutes, and also correction doses, as part of its meal detection technology.
This is an evolution of the 670G, the world’s first artificial pancreas system, which was approved in 2016, and an updated version, 770G, which was authorized in the United States in 2020. (The 780G was approved in Europe in 2020.)
In the pivotal clinical trial:
- Time-in-range (blood sugar between 70-180 mg/dL) was 75 percent, helping those with T1D maintain more consistent and healthier glucose levels
- The overall time-below-range was 1.8 percent, and overnight it was 1.5 percent
- Overnight time-in-range was 82 percent
The 780G is approved for use with the Medtronic Guardian 4 continuous glucose monitor (CGM). Unlike its predecessor, the Guardian 3, the Guardian 4 does not require fingerstick calibrations.
The 780G is also approved for use with the Medtronic Extended Infusion Set (EIS), which can be used for seven consecutive days (most infusion sets can be used for 3 days).
Per Medtronic, individuals will be able to pre-order the device on May 15 with an expected delivery in June.
Breakthrough T1D Impact
Breakthrough T1D’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, Breakthrough T1D developed a roadmap for artificial pancreas development with projections for next generation versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- Breakthrough T1D started the Artificial Pancreas Project almost 20 years ago to ensure that people with T1D would have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The Breakthrough T1D Artificial Pancreas Project and the Breakthrough T1D Artificial Pancreas Consortium have dramatically accelerated progress by bringing together academic researchers, government agencies, industry partners, and the Helmsley Charitable Trust to pursue artificial pancreas technology.
Breakthrough T1D has funded over $140 million to date in artificial pancreas research.
- Five algorithm teams of the Breakthrough T1D Artificial Pancreas Consortium and Tidepool developed algorithms that made the Medtronic MiniMed 670G, 770G, and 780G, Tandem Control-IQ™, CamAPS® FX, Insulet Omnipod 5, and Tidepool Loop
- Industry experts have said Breakthrough T1D’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system, the first artificial pancreas system, which was approved in 2016.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management until cures are found.
Cynthia Rice, Breakthrough T1D’s Chief Mission Strategy Officer, will leave behind quite a legacy once she steps down from her role at Breakthrough T1D at the end of March 2023.
“In her time with Breakthrough T1D, she has led with strategic purpose and passion,” read Breakthrough T1D CEO Aaron Kowalski’s December 2022 memo announcing Cynthia’s decision to leave. “She has been an incredibly valuable partner to me, as well as staff and volunteers throughout the organization.”
During her tenure at Breakthrough T1D, she has helped bring the artificial pancreas project to life, has driven efforts to renew the Special Diabetes Program, and was a key player in Breakthrough T1D’s response to and handling of COVID-19—all with the partnership of our strategic staff and vast network of volunteers, who are the bedrock of our advocacy efforts.
“It’s possible—while challenging—to impact the research and development (R&D) ecosystem to improve options and outcomes for people living with chronic diseases like type 1 diabetes,” says Cynthia. “Defining goals, tapping into strengths, building capacities, and remaining determined in the face of obstacles are critical.”
And for nearly two decades, she has done just that at Breakthrough T1D.
“Leverage—enlisting others to our cause—is critical to our success and core to our organizational DNA, whether it’s engaging friends and families, company R&D heads, government officials, or foundation leaders,” Cynthia says.
The Artificial Pancreas Endeavor

From Left: Breakthrough T1D International Board of Directors member Claudia Graham, Ph.D., M.P.H.; Breakthrough T1D Chief Mission Strategy Officer Cynthia Rice; and Senator Susan Collins (R-ME). Left-click on image to slightly enlarge.
Cynthia came to Breakthrough T1D in September 2005. Real-time continuous glucose monitors (CGMs) were in the early stages of development, with one approved just months prior.
Aaron Kowalski, Ph.D., who had come to Breakthrough T1D a year before and is now the CEO, and Jeffrey Brewer, a member of Breakthrough T1D’s International Board of Directors at the time, had just spent six months interviewing academic scientists, corporate executives, and other like-minded players to figure out whether Breakthrough T1D wanted to take on the development of an artificial pancreas. There were many barriers, and companies were very wary of getting involved.
In the interviews, it became clear that despite the hesitation among companies, there was significant potential benefit for the T1D community in pursuing artificial pancreas technologies. The leadership—needed to foster a therapy roadmap, research funding, regulatory pathway, and health care access—just wasn’t there.
Breakthrough T1D changed that. We made it a priority, bringing not only our research funding, but also our powerful advocacy forces, to speed the development of these devices.
“The goal of multiple artificial pancreas systems, with ongoing innovation, drove our strategy,” says Cynthia, “and we took actions early on with that goal in mind, utilizing our strengths, building new capacities and relationships, and battling doggedly to overcome obstacles.”
Overcoming the Obstacles
Among the first obstacles was that the benefits of continuous glucose monitoring in the management of T1D had only been shown in small studies. In 2008, a Breakthrough T1D multi-site randomized control clinical trial showed that people with T1D who used the devices experienced significant improvement in blood-sugar control. This was instrumental to CGM coverage and laying the groundwork for the artificial pancreas system to come to fruition and be covered by the healthcare system.
Another obstacle was linking together the two main components of a closed-loop artificial pancreas system—the glucose sensor and an insulin pump. Breakthrough T1D established the Artificial Pancreas Consortium, which connected researchers from several different laboratories to develop the computer algorithms so that the machines could “talk” to each other and then be commercialized, as necessary.
A third obstacle—perhaps the most challenging of them all—required engaging government, regulatory, and health care groups.
Breakthrough T1D worked with researchers, insurance companies, the National Institutes of Health (NIH), the U.S. Food and Drug Administration (FDA), Medicare, and Congress on regulatory and coverage issues. When the first artificial pancreas system came on the market in 2016, the T1D community was more than ready for the life-changing T1D management it offered.
“Seeing the artificial pancreas go from concept to reality, which is helping so many people keep their blood-sugar management in control, is what makes Breakthrough T1D and all of the advocacy volunteers—who sent an email, made a call, signed an action alert, or met with their Member of Congress—very proud of this historic achievement and the impact that these will have on the individual lives of those with type 1 diabetes,” Cynthia adds.
The Special Diabetes Program (SDP)

Cynthia Rice (far right, fifth row from the back), Breakthrough T1D volunteers, and Delegates at Children’s Congress 2013 with then-Vice President of the United States Joseph R. Biden (center). Left-click on image to slightly enlarge.
In 1997, with the bipartisan leadership of White House Chief of Staff Erskine Bowles and Speaker Newt Gingrich, Congress created the Special Diabetes Program (SDP), which annually allocates $150 million for T1D research at the NIH. Breakthrough T1D is the chief advocate of the SDP.
The SDP has been instrumental to some of the greatest advancements in the history of T1D—including research that led to artificial pancreas systems and the recent FDA approval of the first-ever drug that can delay onset of T1D, Tzield™ (teplizumab-mzwv).
Since its inception, the SDP has invested $3.4 billion into T1D research. The program’s success and continued renewal is the result in part of hundreds of Breakthrough T1D advocates meeting with their Members of Congress each year to discuss the importance of the SDP.
“Sustaining bipartisan support to renew again and again in challenging times in Washington is thanks to the amazing volunteer-staff partnership in advocacy,” says Cynthia. “This is now paying enormous dividends, not only in the artificial pancreas systems, but in cures therapies, including disease-modifying and cell therapy treatments.”
Breakthrough T1D’s Unique Strengths
“Breakthrough T1D has two strengths that are rare,” says Cynthia. “The first is scientific expertise, convening the best and brightest across fields and generating ideas to solve the biggest problems. The second is community passion, to influence R&D priorities, regulatory pathways, and health care access and enlist government leaders to take action for our cause.”
She adds: “Breakthrough T1D has harnessed these strengths and organized the community, leading to our higher-level power: Influence.”
“It’s not only possible but realizable for a small band of determined people, starting with our founding moms, to tackle and overcome big obstacles,” says Cynthia. “As long as we organize ourselves well, deploy smart strategies, and develop an advocacy message that people can get around, Breakthrough T1D will continue to have the impact that has historically been the pillar of our advocacy work.”
“More broadly, strong patient advocacy strengthens our health system and our society and helps align incentives in research, development, and health policy to benefit the people affected by the disease,” says Cynthia. “All of us as leaders should be thinking about what else we can do to help strong, independent patient communities come together and thrive as advocates, which I hope to do when I return to the health sector in 2024 after a sabbatical.”
A Legacy of Women Leaders
Breakthrough T1D was founded by women, has mostly women staff and volunteers, and counts numerous successful and influential women among its current and past leaders and supporters.
Women who, like Cynthia Rice, share Breakthrough T1D’s vision for a world without T1D and who will stop at nothing to turn that vision into reality.
“From the majority staff and volunteer base, to our women founders, to our international chair Mary Tyler Moore, to our advocates, fundraisers, and scientists,” says Cynthia, “Breakthrough T1D, as an organization, shows the power women can have to impact their world.”

Members of Breakthrough T1D’s Grassroots Leadership Team (GLT) along with members of Breakthrough T1D’s Advocacy Team, including Cynthia Rice (ninth person in from the right) at Breakthrough T1D Government Day 2023. Left-click on image to slightly enlarge.
